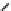Chemistry Reference
In-Depth Information
ΔS > 0
ΔS´< ΔS
Figure 5.5:
Sketch explaining the increase in melting point with increase in chain rigidity.
29
Reproduced by permission of John Wiley and Sons.
chain will not be degraded into two shorter ladder structures except in the
unlikely event that two single-chain scissions occur directly across from
one another.
91
There is considerable interest in inserting a silphenylene group [-
Si(CH
3
)
2
C
6
H
4
-] into polysiloxane backbones.
29,
92-98
In the case of the PDMS
repeat unit, insertion yields the
meta
and
para
silphenylene polymers
shown in figure 5.6.
29
he
T
g
is increased to -48°C compared to -125°C for
PDMS, but no crystallinity has been detected. Since the repeat unit is sym-
metric, it should be possible to induce crystallinity by stretching. The expla-
nation here is the same as that given in figure 5.5 except that the chains are
prevented from completely disordering by the stretching force, rather than
T
g
= -48
°
C
T
m
= ?
O
Si
Si
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
T
g
= -18
°
C
T
m
= 148
°
C
Si
O
Si
CH
3
CH
3
Figure 5.6:
Meta
and
para
silphenylene polymers and their transition temperatures.
29
Reproduced by permission of John Wiley and Sons.