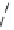Chemistry Reference
In-Depth Information
CH
2
CH
2
CH
2
Si
Si
CH
3
CH
3
CH
3
CH
3
Figure 5.2:
The poly(dimethylsilmethylene) chain.
17
Reproduced by permission of the American
Chemical Society.
5.2.4 Poly(dimethylsilmethylene)
Poly(dimethylsilmethylene), [-Si(CH
3
)
2
-CH
2
-]
x
, can be thought of either
as a hydrocarbon analogue of PDMS (in which O atoms are replaced by
CH
2
groups) or as a silicon analogue to polyisobutylene [-C(CH
3
)
2
-CH
2
-]
x
(in which Si atoms replace one of the two skeletal C atoms in the repeat
unit).
17
Figure 5.2 shows the polymer schematically. The Si-C bonds are
1.90 Å and, in contrast to siloxane chains, the two types of skeletal bond
angles are essentially identical and tetrahedral. Since CH
2
and CH
3
groups
have very similar interactions, this chain molecule should have some
characteristics reminiscent of the idealized “freely rotating” chain.
16, 20, 21
This conclusion is supported by experimental evidence, which indicates
that the characteristic ratio of the polymer is relatively small and that
both its unperturbed dimension and dipole moment are essentially inde-
pendent of temperature.
17
5.3 FLEXIBILITY OF THE POLYMER CHAINS
5.3.1 Equilibrium Flexibility
Equilibrium flexibility has a profound effect on the melting point,
T
m
, of a
polymer. Since crystallites in turn have a profound effect on the proper-
ties, the crystallization PDMS has been a subject of considerable activ-
it y.
23, 79-82
The melting points of small molecules in PDMS networks have
also been reported.
83
High flexibility in the equilibrium sense means high conformational
randomness in the amorphous state, and thus high entropy of fusion and
low melting point. This entropy can be reduced by stretching, in what is
called “strain-induced crystallization.” Such strain-induced crystalliza-
tion has been studied extensively, both experimentally
84-85
and theoreti-
ca l ly.
86-87
The crystallites thus generated can be very important since they