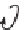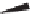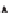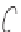Chemistry Reference
In-Depth Information
O
O
O
Si
Si
CH
2
CH
2
CH
2
CH
3
CH
2
CH
3
Figure 5.1:
The poly(di-
n
-propylsiloxane) chain, showing the conformational variability of the propyl
side chains.
17
Reproduced by permission of the American Chemical Society.
variable polysiloxanes (see section 2.2) suggest that the characteristic
ratio increases with increase in length or size of the side groups.
Cyclization studies have also been carried out on the chemical copoly-
mers poly(ethylene, dimethylsiloxane) and poly(styrene, dimethylsilox-
ane).
36-38
Numerous intramolecular interactions need to be taken into
account in a chemical copolymer. Consequently, the results on the copoly-
mers have been given only a preliminary interpretation in terms of rota-
tional isomeric state theory. Cyclization calculations have also been
carried out for poly(dihydrogensiloxane) [-SiH
2
O-]
x
, but at present there
are no experimental data available for comparison with theory.
Finally, melting point depression measurements have been conducted
on several symmetrically substituted polysiloxanes, specifically the di-
methyl, diethyl, di-
n
-propyl, and diphenyl polymers. Interpretation of
such experimental results yields entropies of fusion. Although it is diffi-
cult to extract a reliable configurational entropy from this quantity, such
results could help elucidate the configurational characteristics of the
chains thus investigated.
17
5.2.2 Stereochemically Variable Polysiloxanes
In unsymmetrically disubstituted chains, the substituents of one type can
be on the same side of the all-
trans
chain, on opposite sides, or on either
side in a random arrangement, yielding isotactic, syndiotactic, and atactic
forms. Poly(methylphenylsiloxane) was one of the chains chosen to illus-
trate this stereochemical variability. The relatively large Si-O bond length
and Si-O-Si bond angle place apposed side groups at distances of separa-
tion (ca. 3.8 Å) at which there is a favorable energy of interaction.