Geology Reference
In-Depth Information
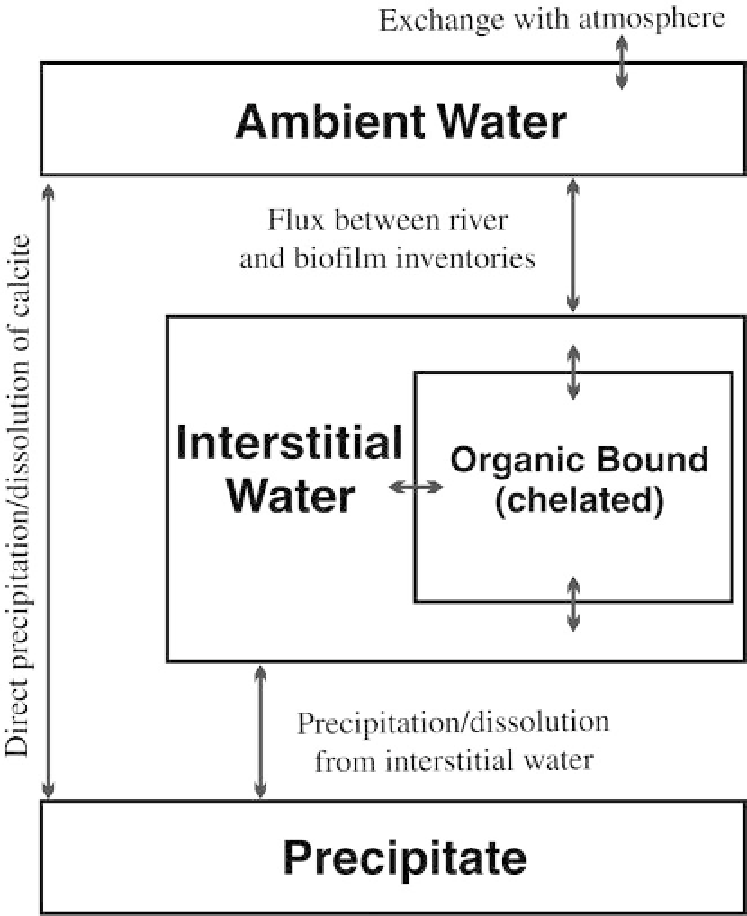
Fig. 10. A conceptual four-phase model for dissolved ions within a tufa system, showing pathways of mass exchange.
2008a, b; Shiraishi et al. 2008a) provides an expla-
nation for this, supporting a biomediated view
for tufa precipitates. Consequently, rather than con-
sidering tufa systems a two-phase system (i.e.
water-calcite) a process-oriented understanding
of precipitation will require consideration of the
four-phase system outlined in Figure 10. In this
context, our conductivity data should be seen as
indicating flux of ions into/out of the Ambient
Water inventory and field data seen as point ana-
lyses of the state of this part of the tufa system.
In a recent study in which the hydrochemistry of
a pool within a Chinese tufa forming stream was
monitored over a 48 hour period, Liu et al. (2008)
show that pH varies by 0.8-1 on a diurnal timescale,
and that this change was dominantly driven by the
balance of photosynthesis and respiration. More-
over, via conductivity measurements they demon-
strate that precipitation of calcite occurs during
daytime pH maxima, and a degree of dissolution
occurs during night time pH minima. Our results
endorse this view, and comparison of the data
provide significant new insights into the role of
photosynthesis in promoting precipitation. Unlike
the data presented here, the Chinese study show
pH (conductivity) fairly smoothly increasing
(decreasing) towards midday followed by a fairly
smooth decrease (increase) back to night time
values. This different character is a consequence
of the different lighting regimes, as unlike natural
sunlight our lamps are either on or off. Unfortu-
nately, Liu et al. (2008) do not present light intensity
Search WWH ::

Custom Search