Geology Reference
In-Depth Information
those obtained by previous studies for both gram-
negative and -positive bacteria and their polymeric
substancies, see Tourney et al. 2008 for a review.
The results presented here indicate that polysac-
charides from cyanobacteria have a strong potential
to exchange protons with their surrounding environ-
ment. The amount of polysaccharides produced in
cultures of the three strains tested shows that this
component cannot be neglected.
Cyanobacteria are often habited calcified mats
with extreme daily fluctuations in geochemical con-
ditions, for example, typical variations in pH from 8
to 9 during day-night time (Shiraishi et al. 2008).
Under such circumstances, sulphydric and amine
groups will periodically change their protonation
states, releasing protons, when the pH increases,
and binding protons, when pH decreases. Therefore,
the functional groups with pK
a
values from 7-9 will
contribute to the buffer capacity and also the alka-
linity balance, and therefore, influence the satur-
ation index of carbonate.
It is interesting to note that our titration and FTIR
data suggest the presence of the sulphur-containing
groups. The degradation products of these groups
may act as the energy and carbon sources for anaero-
bic heterotrophs (Lovley & Coates 2000). The inti-
mate coupling of C- and S-cycles in the mat through
metabolic activity of cyanobacteria and SRB has
been suggested to result in the biogenic production
of the sulphur compounds that represents an impor-
tant source of volatile compounds typically emitted
from mats and greatly impact the Earth's atmos-
phere (Visscher et al. 2003). Our study showed
one possible link between cyanobacteria and SRB
through the degradation of cyanobacterial poly-
saccharides under the fluctuating geochemical con-
ditions in mats.
CaCO
3
precipitation by polysaccharides
CaCO
3
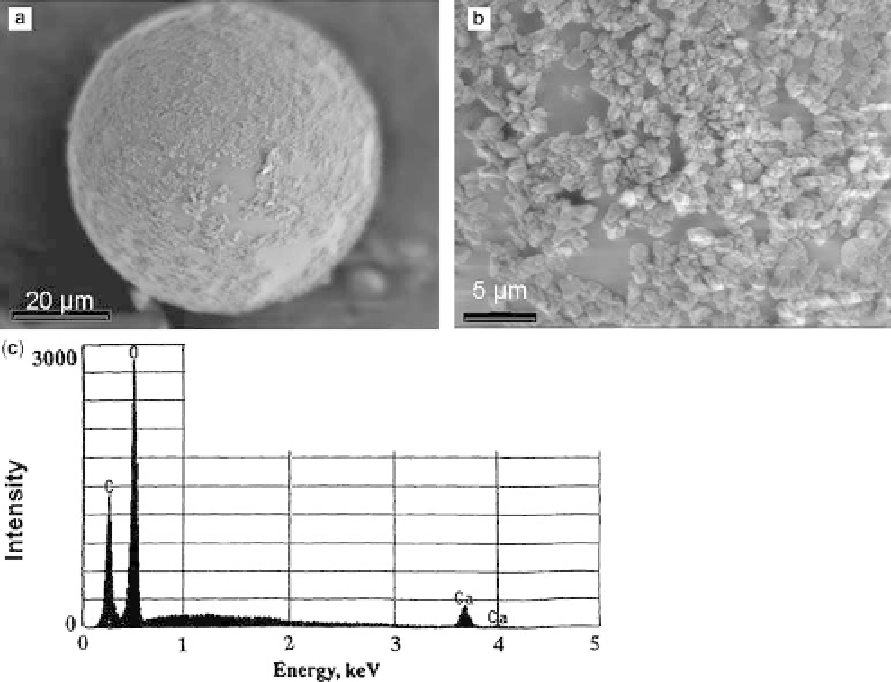
minerals were present on the surface of
polysaccharides-coated agarose beads after 5 days
of incubation (Fig. 5a, b, c). In controls lacking
EPS, CaCO
3
minerals were not observed (Fig. 6a,
b). The presence of calcium carbonate in the
Fig. 5. Scanning electron microscopy images of coated agarose beads after CaCO
3
precipitation experiments.
(a) Spherical bead with small rhombohedral precipitates on surfaces. (b) Close-up of rhombohedral precipitates.
(c) EDX spectrum of precipitates which is typical for CaCO
3
.
Search WWH ::

Custom Search