Biomedical Engineering Reference
In-Depth Information
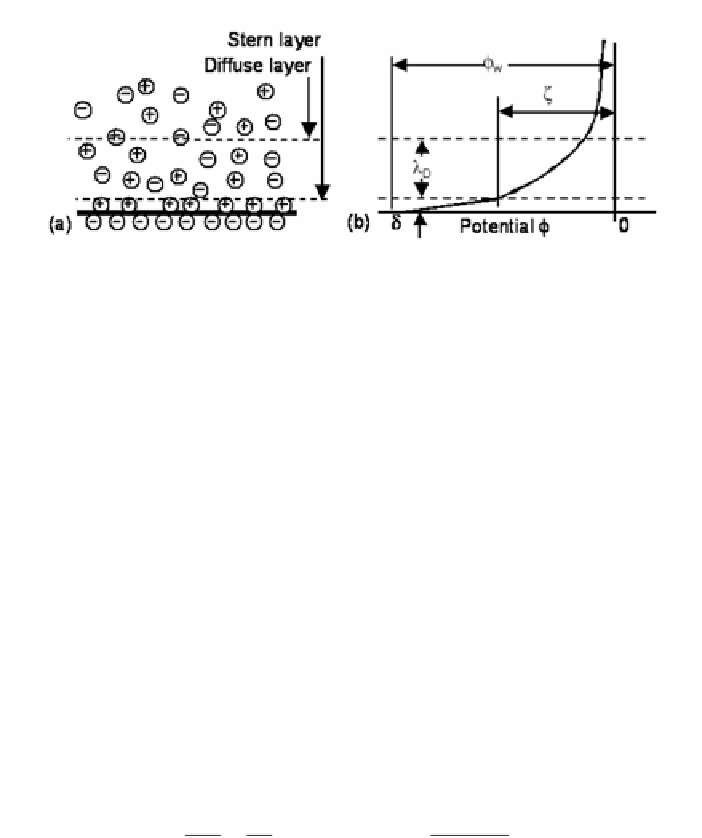
Figure 1
. Sketch of the electric double layer showing (
a
) the Stern layer and the diffuse layer
and (
b
) the resulting potential.
surface chemistries, which means that the EDL is positively charged. The gov-
erning equation for the electric potential G is found to be the Poisson-Boltzmann
equation:
d
2
G
2
Fzc
zF
G
=
d
sinh
,
[1]
dy
2
F
KT
where
c
is the concentration of ions far from the surface,
z
is the charge number
(valence) of each ion, F = F
r
F
0
is the dielectric constant of the liquid, G is the
electric potential,
T
is the absolute temperature,
K
is Boltzmann's constant, and
F
is Faraday's constant. This equation is clearly nonlinear and difficult to solve.
However, the relative thickness of the EDL is usually small enough in micron-
sized systems that the hyperbolic sine term can be replaced by the first term in
its Taylor series—just its argument. This approximation is called the
Debye-
Hückel limit
of thin EDLs and it greatly simplifies Eq. [1] to
d
2
GG
F
KT
2
=
where
M
=
,
[2]
D
dy
2
M
2
2
z F c
2
2
D
d
where M
D
is called the
Debye length
of the electrolyte. The solution to this ordi-
nary differential equation is quite straightforward and found to be
¬
-
y
-
GG
=
exp
--
®
.
[3]
w
M
D