Environmental Engineering Reference
In-Depth Information
compared with mono-silanes due to the double number
of hydrolyzable OR groups [22, 30].
Bis-silanes improve the anti-corrosion performance by
depositing a more hydrophobic layer (SiOSi) on the sub-
strate's surface during the condensation, and also through
chemical bonding of the functional groups present in the
bis-silane itself or with a mono-silane solution additive. It
was observed that a mixture of silanes performed better cor-
rosion protection than a single silane [22, 31]. Unfortunately,
most of the bis-silanes are not soluble in water, limiting their
industrial applications. Nevertheless, in optimal mixtures it
could be proven that silanes are able to ensure equivalent
OR
OR
Si
OR
OR
Si
OR
OR
Figure 2.2
Chemical structure of bis-silane.
Table 2.2 Formula and chemical structure of typical bifunctional silanes.
Name of silane
Formula
Chemical structure
bis-1,2-
[triethoxysilyl
propyl] ethane
(BTSE)
CH
3
CH
3
O
H
3
C
(OC
2
H
5
)
3
-
Si(CH
2
)
2
Si(OC
2
H
5
)
3
O
O
Si
Si
O
O
CH
3
O
H
3
C
H
3
C
bis-1,2-
[triethoxysilyl
propyl]
tetrasuli de
(BTESPT)
CH
3
CH
3
O
H
3
C
(OC
2
H
5
)
3
Si(CH
2
)
3
S
4
(CH
2
)
3
Si(OC
2
H
5
)
3
O
O
S
S
O
Si
S
S
Si
CH
3
O
O
H
3
C
H
3
C
bis-[1,2-trime-
thoxysilylpro-
pyl] amine,
BTSPA
CH
3
CH
3
O
H
3
C
(OCH
3
)
3
Si(CH
2
)
3
NH
(CH
2
)
3
Si(OCH
3
)
3
O
O
NH
Si
Si
O
O
O
CH
3
CH
3
CH
3










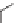



















































Search WWH ::

Custom Search