Environmental Engineering Reference
In-Depth Information
oxygen vacancies or oxygen interstitial, zinc vacancies or oxygen intersti-
tial, or a complex of these native defects. h e concentration of a defect in a
crystal depends upon its formation energy E
f
in the following form [111]:
f
−
E
CN
=
exp
16.4
sites
KT
B
where N
sites
is the concentration of sites in the crystal where the defect can
occur, while formation energy of a point defect in a charge state q can be
written as:
total
f
Eq E qn nμ qE
()
=
()
−
−
−
(16.5)
Zn Zn
OO
F
where E
total
(q) is the total energy of a system containing n
Zn
and n
O
num-
ber of zinc and oxygen atoms, μ
Zn
and μ
O
are the chemical potentials for
zinc and oxygen, respectively, and E
F
is the Fermi energy [3]. h e chemical
potential is dependent upon the growth condition and decides formation
energy of a defect, and low formation energy defects reveal high defect con-
centration in crystal. In zinc-rich growth condition, oxygen vacancies have
lower formation energy, while zinc vacancies have lower formation energy
in oxygen-rich condition. Oxygen vacancies can be formed in neutral, +1
or +2 charge state (V
o
, V
o
+1
and V
o
+2
), and zinc can have 0, -1 and -2 charged
defect states in ZnO crystal. h ough native point defect has low forma-
tion energy and highly dominates in structure, some other defects like grain
boundaries, line defect or plane defect can also be present in the ZnO sys-
tem. Grain boundaries are very signii cant defect in nanostructured thin
i lms; a depletion region is generally formed at each grain boundary and
strongly af ects optical and electrical properties [112]. h reading disloca-
tions of 20
from the c-axis are also observed in plasma-assisted MBE
grown ZnO under Zn-rich conditions [113]. h ese dislocation transmis-
sions may have negative charge due to the accumulation of Zn vacancies
near the dislocation core [112]. It is observed that dislocations created by
plastic deformation increase the excitonic emission intensity [114]. Basal-
plane stacking fault is seen in MBE grown ZnO i lm on sapphire, but no
electronic states creation is observed [115, 112]. Some other impurity ele-
ments/unintentional dopant and intentional dopant can create defects in
the ZnO crystal. Nitrogen can substitute oxygen and exists in two distinct
chemical environments, attributed to N acceptors and N
2
molecules [116].
Phosphorus, arsenic, antimony, lithium, sodium and copper are studied as
°
- 30
°



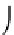
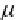

Search WWH ::

Custom Search