Environmental Engineering Reference
In-Depth Information
Br
Br
Br
Br
Br
Br
O
-
O
-
OH
O
O
O
-H
+
+H
+
Br
Br
Br
Br
Br
Br
SO
3
-
SO
3
-
SO
3
-
Figure 15.5
h e resonance structure of Bromocresol green.
reaction mechanisms [41, 86]. h e photocatalytic activity of a semicon-
ductor is largely controlled by: (i) the light absorption properties, e.g., light
absorption spectrum and coei cient, (ii) reduction and oxidation rates on
the surface by the electron and hole, and (iii) the electron-hole recombina-
tion rate [86].
15.3
Synthetic Methods for Novel Titania
Photocatalysts
h ere are dif erent preparative methods described in the literature to obtain
the preparation of nanosize transition metal-doped titanium dioxide pho-
tocatalysts. h e widely known methods to prepare nanomaterials com-
pound are: sol-gel synthesis and novel chemical method. h e precursor
routes play a crucial role in designing the i nal products and are also bet-
ter and more convenient methods for the preparation of multicomponent,
transition metal-doped titanium dioxide nanophotocatalysts. h e precur-
sor compounds are usually complex combinations of cations in the proper
ratios, together with ionic and molecular species, containing the necessary
oxygen for the formation of solid solutions or the crystalline compounds.
h e remainders are volatile, hence are decomposable into volatile elements.
h e pyrolysis of the complex combinations at temperatures between 200
°
C
to 500
C in an appropriate inert/oxidizing/reducing atmosphere, depend-
ing on the material, gives nanoscale particles of the desired mixed-oxide
system with a good cation stoichiometry. Literature reveals that various
organometallic complexes (metal alkoxides, etc.), metal-hydroxides/-
carbonates/-oxalates/-citrates/-nitrates, are a few of the commonly used
precursor compounds in this process [87-88]. Very recently, Pramanik
et
al.
prepared the nanosized transition metal-doped titanium dioxide pho-
tocatalyst by precursor decomposition method [26, 41, 89].
h e precursor method involves preparation of a precursor compound.
Morgan [90, 91] has suggested that the use of chemistry in the preparation
°

















































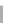



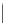


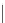



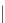








Search WWH ::

Custom Search