Environmental Engineering Reference
In-Depth Information
been devoted to the synthesis of magnetic nanoparticles. Especially dur-
ing the last few years, many publications have described ei cient synthetic
routes to shape-controlled, highly stable, functionalized, and monodis-
perse magnetic nanoparticles. h ese methods have been used to prepare
particles with homogeneous composition and narrow size distribution.
However, the most common method for the production of magnetite
nanoparticles is the chemical co-precipitation technique of iron salts.
11.5.1 Co-precipitation
Co-precipitation is a very facile and convenient way to synthesize iron
oxide nanoparticles (either Fe
3
O
4
or γ-Fe
2
O
3
) from aqueous Fe
2+
/Fe
3+
salt
solutions by the addition of a base under inert atmosphere at room tem-
perature or at elevated temperature. h e chemical reaction of Fe
3
O
4
forma-
tion may be written as [65]:
2
+
3
+
−
Fe
+
2
Fe
+
8
OH
Fe O
+
4
H O
(11.1)
34
2
According to the thermodynamics of this reaction, complete precipita-
tion of Fe
3
O
4
should be expected at a pH between 8 and 14, with a stoi-
chiometric ratio of 2:1 (Fe
3+
/Fe
2+
) in a non-oxidizing oxygen environment.
h e size, shape, and composition of the magnetic nanoparticles very much
depend on the type of salts used (e.g., chlorides, sulfates, nitrates), the Fe
2+
/
Fe
3+
ratio, the reaction temperature, the pH value and ionic strength of
the media. With this synthesis, once the synthetic conditions are i xed, the
quality of the magnetite nanoparticles is fully reproducible. However, mag-
netite nanoparticles are not very stable under ambient conditions, and are
easily oxidized to maghemite or dissolved in an acidic medium.
+
2
+
Fe O
+
2
H
−
Fe O
+
Fe
+
H O
(11.2)
34
24
2
Oxidation of maghemite is the lesser problem, as it is a ferrimagnet.
h erefore, magnetite particles can be subjected to deliberate oxidation to
convert them into maghemite. h is transformation is achieved by dispers-
ing them in acidic medium, then adding iron(III) nitrate. h e maghemite
particles obtained are then chemically stable in alkaline and acidic medium
[66]. h e main advantage of the co-precipitation process is that a large
amount of nanoparticles can be synthesized in a short time. However, the
control of particle size distribution is limited, because only kinetic factors
are controlling the growth of the crystal [65]. In the syntheses of magnetic
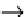
Search WWH ::

Custom Search