Environmental Engineering Reference
In-Depth Information
Au(111) precovered with 0.4 ML atomic oxygen when prepared by decom-
position of O
3
at 200 K [173].
8.5.1
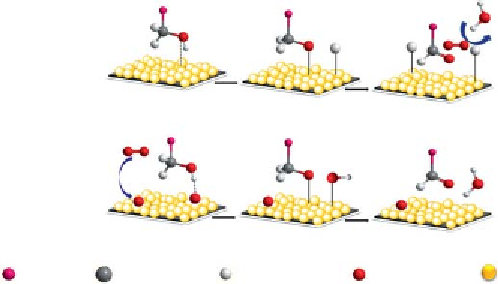
Mechanism for Alcohol Oxidation Reaction
h e mechanism of gold-catalyzed alcohol oxidation by using molecular
oxygen was followed by a multi-step mechanism given by Boronat
et al.
[174] (Scheme 8.9). Initially deprotonation of the hydroxyl group form-
ing a metal-alkoxide intermediate and then β-hydride elimination took
place yielding the carbonylic product. Oxygen reacts with the hydrogen
atoms to produce H
2
O or participates in the i rst step of the mechanism
by assisting the deprotonation of the alcohol. In the case of metal oxide-
supported gold nanoparticles catalysts, the alkoxide intermediate is prob-
ably formed on the support or at the metal-support interface. But when
the reaction is catalyzed by naked gold nanoparticles in solution, stabilized
by polymers, or supported on carbon or SiO
2
, activity has been related to
the presence of low coordinated atoms placed at corner or edge positions
[110, 175-177]. However, the ef ect of chemisorbed basic O atoms is con-
siderably more important and decreases the activation energy for depro-
tonation of the hydroxyl group by
20 kcal/mol, while it has no inl uence
on the dissociation of the C-Hβ bond [178]. It can then be concluded that
alcohol oxidation is favored by the presence of chemisorbed oxygen atoms
able to abstract protons and dissociate the hydroxyl group. h ese basic
oxygen atoms are stabilized on 3D gold nanoparticles and not on small
planar clusters.
∼
(R Group)
(Carbon atom)
(Hydrogen atom)
(Oxygen atom)
(Gold nanoparticle)
Scheme 8.9
Alcohol oxidation reaction on supported gold nanoparticles catalyst.
Search WWH ::

Custom Search