Environmental Engineering Reference
In-Depth Information
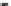
(a) CeO
2
(b) Sm-CeO
2
(Sm/Ce = 4/100)
(c) Au/Sm-CeO
2
(Sm/Ce = 4/100)
0.35
0.30
0.25
0.20
0.15
0.10
0.05
0.00
0
100
200 300 400
Temperature (
0
C)
500
600
700
800
Figure 8.30
H
2
-TPR of the catalysts. Reproduced with permission from [161].
as an ef ective support for gold nanoparticles. h ey investigated the role
of dif erent types of dopant like samarium, manganese and dif erent alkali
metal like Ba
2+
, Ca
2+
and Mg
2+
on cerium oxide material [161-163]. In their
investigation they showed that gold nanoparticles preferably nucleate on
the oxide vacant site and these supported gold nanoparticles which facili-
tate the easy reducibility of the catalyst.
Alcohol oxidation over Au/CeO
2
catalyst following a complex multi-
step mechanism was given by Corma
et al.
[158]. According to this mecha-
nism (Scheme 8.7), the interaction between gold and ceria will give rise to
an important population of positively charged gold and Ce
3+
species. h e
alcohol(s) (Scheme 8.8) or the surface adsorbed alkoxide will then react
with the Lewis acid sites of Au/CeO
2
to give a metal alkoxide, which sub-
sequently undergoes a rapid hydride transfer from C-H to Ce
3+
and Au
+
to
give the ketone and Ce-H (indicated as LA-H) and Au-H. Upon admission
of oxygen into the system and coordination to the oxygen-dei cient sites
of ceria, formation of cerium-coordinated superoxide (Ce-OO
) species
occurs [164]. h e increment of oxide vacant site on support material facili-
tates the formation of cerium-coordinated superoxide (Ce-OO
) species.
h ese superoxide species evolve into cerium hydroperoxide by hydrogen
abstraction from Au-H, and are responsible for the formation, at er reduc-
tion of Ce(IV), of the initial Au
+
species. h e absence of gold would render
this step impossible and lead to a depletion of Ce(III).





















































Search WWH ::

Custom Search