Environmental Engineering Reference
In-Depth Information
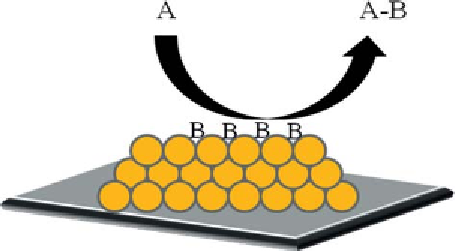
Scheme 8.3
Eley-Rideal mechanism
reaction order remains constant at a value near 0.9. h ey conclude that
with rising glucose concentrations, glucose successfully competes with
oxygen for adsorption sites, leading to lower oxygen coverage and, thus, to
a higher reaction order for oxygen.
To know the reaction mechanism we have to analyze the stepwise path-
way of the reaction. From the literature it was found that Pina
et al.
[95] try
to explain the kinetic mechanism of “naked” gold particles as a catalyst for
glucose oxidation. h ey found that low glucose concentrations (< 0.1 M)
show a i rst order reaction; the assumed higher concentration is 0.5 M. h e
reaction follows the Eley-Rideal mechanism [115] (Scheme 8.3).
h is mechanism gives rise to a rate equation that justii es both the i rst
order with respect to oxygen and the decreasing order with respect to glu-
cose, being i rst order at low concentration and tending to zero for large
values.
A similar observation was reported by Comotti
et al.
[97]. Kinetic
tests were carried out employing unsupported colloidal gold particles as
“naked” particles; it was found that the fundamental detection of hydro-
gen peroxide, instead of water, was the reduction product of dioxygen.
From Scheme 8.4 it can be seen that the rate determining step of the gold-
catalyzed glucose oxidation reaction is the adsorbed glucose which is oxi-
dized by dioxygen dissolved in water, according to a i rst order dependence
of the reaction ratevon pO
2
.
h e fundamental step is the formation of the electron-rich gold species
derived by the hydrated glucose anion with gold. h is species is supposed
to activate oxygen by nucleophilic attack, and the derived dioxo-gold inter-
mediate can then behave as a bridge for the two electrons transfer from
glucose to dioxygen.
Search WWH ::

Custom Search