Environmental Engineering Reference
In-Depth Information
Au foil
Au/Ti-SiO
2
(3/100) fresh
Au
2
O
3
11850
11900
11950
12000
12050
Energy / eV
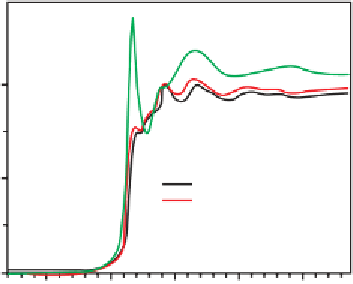
Figure 8.14
XANES spectra of
Au L
3
-edge spectra of the
Au/Ti-SiO
2
catalyst.
these hydroperoxo intermediates have been studied in depth by a plethora
of experimental techniques [81-84]. Recently, spectroscopic studies have
been found in the literature showing the formation of Ti-peroxo/hydro-
peroxide species for Pd supported over dif erent types of Ti-containing
zeolites [85], and also for Ti-SBA-15 material [86] in the presence of liquid
H
2
O
2
. It has also been found that organic hydroperoxides in liquid phase
form a peroxo complex analogous to the hydroperoxo species formed by
hydrogen peroxide [36].
Based on experimental results and literature reports a mechanistic
model for the formation of superoxo/hydroperoxo species on gold surface
is shown in Figure 8.15. h e potential ability of Au to form peroxo-type
species has been postulated [87] and experimentally verii ed [88-91]. As
per this model, formation of hydrogen peroxide from H
2
and O
2
is initiated
on gold surface.
Once H
2
O
2
is generated it can be transferred into hydroperoxo species
on tetrahedrally coordinated Ti cation sites, and then react with propylene
adsorbed on SiO
2
surfaces to form PO. h e Ti-hydroperoxo species, which
is UV active, and Ti-OO
-
species, which is ESR active, can be detected
directly as reported by Chowdhury
et al.
[72, 92] and shown in Figure 8.16.
h e presence of both the species in large amounts in the presence of gold
emphasizes the vital role of gold on Ti-hydroperoxo species formation,
which is indispensable for production of PO from propylene (Figure 8.17).
h e work by Bravo
et al.
[93] shows the formation of Ti-hydroperoxo spe-
cies by
in-situ
XANES spectroscopy.
Search WWH ::

Custom Search