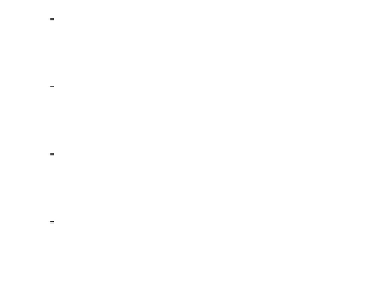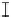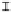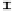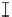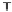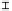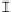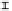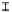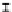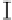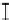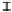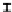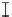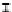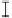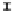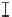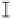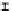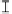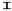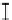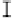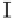Environmental Engineering Reference
In-Depth Information
9
soil A
9
soil B
7
7
citric a cid
oxa lic a cid
ma lic a cid
a cetic a cid
citric a cid
oxa lic a cid
ma lic a cid
a cetic a cid
5
5
3
3
0
0.05
0.1
0.5
1
2
5
10
20
0
0.05
0.1
0.5
1
2
5
10
20
orga nic a cid concentra tion (mmol / L)
orga nic a cid concentra tion (mmol / L)
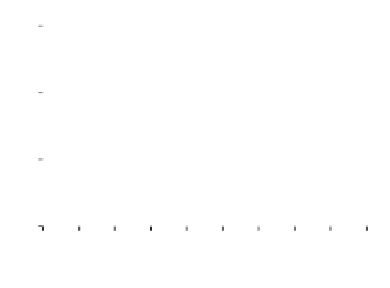
Figure 7. The changes of solution pH with organic acids addition at 0 to 20 mmol/L.
However, there are at least three mechanisms that organic acid induced soil P release: (1)
alter soil pH, (2) compete adsorption sites with P, (3) complex and chelate metal ions which
are bound to P (e.g., Ca-P, Mg-P, Fe-P, and Al-P).
Although organic anions in the soil solution could reduce P adsorption onto the soil's
solid phase, in most situations, P release is mainly controlled by the desorption or dissolution
reaction [15, 18]. The degree of complexation, however, depends on the particular organic
acid involved (number and proximity of carboxyl groups), the concentration and type of metal
and the pH of soil solution [13]. In the two type of studied soils, Ca-P and Mg-P account for a
great proportion in total-P. So Ca and Mg releases were studied synchronically in order to
understand the P activation mechanisms.
At low organic acid concentrations (≤0.5 mmol/L), the P activation capacity of organic
acids in soil B was greatly stronger than that in soil A (Figure 1). Although there were reports
showed that generally high concentrations of organic acids (>100 μ
M
for citrate, >1 m
M
for
oxalate and malate) can mobilize significant quantities of P into soils. However it was highly
dependent on soil type. In some soil, P solubility was highly dependent on soil pH [18, 19]
because complexation of organic acids with metals is highly dependent on soil solution pH.
There appears to be little or no complexation of metals (e.g. Fe and Al) by organic acids at
high soil pH (>8) [1, 13]. The pHs (≈8) of soil A were higher than that (=6-6.9) of soil B, so
metal-complexing ability of organic anions was stronger in soil B than that in soil A, and the
dissolution of Fe-P may increase the P release in soil B with adding low concentrations of
organic acids.
In soil A, soil solution pH generally did not changed with organic acids addition at
different concentrations (0-20 mmol/L) except for citric acid at 20 mmol/L (Figure 7). As a
result, the complexation of organic anions and metal ions may be important for P release form
soil A. Kirk et al. (1999) showed that the main mechanism of P dissolution involved chelation
of metal ions [20]. In soil B, soil acidity effect was more apparent than that in soil A (Figure
7). As organic acids concentration increased, the [H
+
] and complexation effect were greatly
increased, and thereby released Ca bounded P. However, there was little effect on P release
addition acetic acid into soil. Jones showed that organic acids with only one carboxyl group
(acetate) have little metal complex ability [13]. Furthermore, in low soil pH (pH<5), acetic
acid was the main form [21], so P release capacity was low when added high concentration (>
5 mmol/L) of acetic acid in soil B.