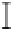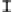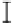Environmental Engineering Reference
In-Depth Information
15
15
oxa lic a cid
citric a cid
13
13
11
11
9
9
7
7
3
4
5
6
7
8
3
4
5
6
7
8
oxa lic a cid pH
citric a cid pH
15
15
13
ma lic a cid
a cetic a cid
13
11
11
9
9
7
7
3
4
5
6
7
8
4
5
6
7
8
ma lic a cid pH
a cetic a cid pH
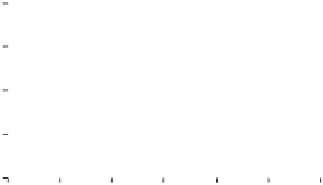
Figure 3. The amount of P extracted by different pH of four organic acids in the alkaline soil solution.
Symbols represent mean ± SEM.
When it comes to citric acid, when pH=8, there was a peak in P concentration but still
lower than citric acid in the initial pH.
3.4. Ca and Mg Desorption Influenced by Concentration of Organic Acids
As is shown in Figure 4, Mg and Ca release capabilities were highly dependent on the
type and concentrations of organic acids and also the type of soils. In soils A and B, the
release trends of Ca were similar with that of Mg. For soil A, the releases of Ca
and Mg from
soil were only significant (
p
<0.01) when organic acids increased to 5 mmol/L. For soil B, Ca
and Mg releases started at 2 mmol/L of organic acids. For oxalic acid, Ca and Mg release
trends were different from the other organic acids which may be resulted from the
precipitation of oxalate with Ca and Mg.
3.5. Ca and Mg Desorption Influenced by Reaction Time
The results revealed a marked difference in Ca and Mg desorption ability between soil A
and soil B as a function of reaction time (Figure 5).
In soil A, there were no obvious changes in Ca and Mg releases when reaction time
varied from 2 to 50 h. However, for soil B, the Ca and Mg releases were generally decreased
with increasing time and reached to a steady state after 24 h. However, for the same
treatments, Ca and Mg releases did not seem to positively relate to P release in both alkaline
and acid solutions.