Environmental Engineering Reference
In-Depth Information
e
-
CB
h
υ
e
-
CB
h
+
VB
Cu
2
O
h
+
VB
Cu
2
O-based cocatalyst
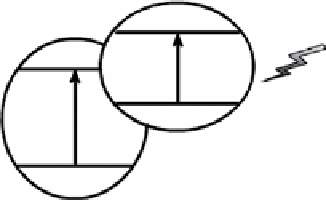
figure 3.10
Scheme of band structure Cu
2
O-based cocatalysts.
reported a solution-based approach to prepare unusual polyhedral 50-facet Cu
2
O microcrystals with a morphological yield
higher than 70%. And the microcrystals prepared in this manner showed a higher specific catalytic rate toward CO oxidation.
Active brilliant red X-3B and
p
-chloronitrobenzene were used as models for the studies on Cu
2
O photocatalytic activity by our
group [134, 138]. The degradation of methylene blue can reach a maximum of 89% after 150-min irradiation due to the synergistic
effect of Cu
2
O and H
2
O
2
[139].
3.3.1.3 Cu
2
O-Based Nanocomposites for Organics Degradation
Cu
2
O-based cocatalysts (such as Cu
2
O/TiO
2
[35, 140-145],
Cu
2
O/ZnO [77, 145, 146], WO
3
/Cu
2
O [147], Cu
2
O/CeO
2
[148], Cu
2
O/BiVO
4
[149], Cu
2
O/Bi
2
O
3
[77], Cu
2
O/SiC [150], Au/Cu
2
O
[80, 81, 151], Ag/Cu
2
O [152], Ag/POM/Cu
2
O [153], Fe
3
O
4
/C/Cu
2
O [154], Cu
2
O/polyaniline (PANI) [155], Cu
2
O nanocube/
polycarbazol [81], Cu
2
O/carbon [156], Cu
2
O nanocubes and multiwall carbon nanotubes with poly tetrafluoroethylene (PTFe)
(Cu
2
O/CNTs/PTFe) [157]) have been extensively investigated for photocatalytic organics degradation. Among these Cu
2
O-
based cocatalysts, bi-semiconductor systems (Cu
2
O/TiO
2
, Cu
2
O/ZnO, WO
3
/Cu
2
O, Cu
2
O/CeO
2
, Cu
2
O/BiVO
4
, Cu
2
O/Bi
2
O
3
, and
Cu
2
O/SiC) have similar band matching structure (Fig. 3.10). The CB of Cu
2
O is higher than that of other semiconductors, while
the VB of Cu
2
O is lower. Thus, photogenerated carriers experience similar transfer process when these bi-semiconductor
systems undergo proper irradiation. The generated electrons in Cu
2
O and holes in counterpart semiconductors migrate to the
CB of counterpart semiconductors and the VB of Cu
2
O, respectively. Thus, the photogenerated electron-hole pairs are sepa-
rated more effectively in the Cu
2
O-based cocatalysts. As for the noble metal/Cu
2
O composite structure (such as Au/Cu
2
O, Ag/
Cu
2
O), the noble metal/semiconductor architecture usually exhibits improved carrier separation and plasmonic enhancement of
VL photocatalytic activity. Three-body composite systems and Cu
2
O/organic composite systems are also found to have an
improved photocatalytic activity for the synergistic effect at work in these compounds. However, we are not going to enumerate
the photocatalytic activity for all Cu
2
O-based co-catalysts because of space constraints. Here, we just want to focus on some
typical Cu
2
O-based cocatalysts: (i) Cu
2
O/wide-gap semiconductor (such as Cu
2
O/TiO
2
, Cu
2
O/WO
3
), (ii) noble metal/Cu
2
O
(such as Ag/Cu
2
O, Au/Cu
2
O), and (iii) Cu
2
O/polymer (such as Cu
2
O/PANI).
3.3.1.3.1 Cu
2
O/Wide-Gap Semiconductor for Organics Degradation
The combination of Cu
2
O and TiO
2
is most extensively
investigated for photocatalytic organics degradation. Our group synthesized Cu
2
O/TiO
2
particle composites and Cu
2
O/TiO
2
composite films for organic pollutant degradation under UV or VL. In 2004, we [75] synthesized nanosized Cu
2
O/TiO
2
particle
composites by an electrochemical method and found that the photocatalytic activities of the composites prepared by this
method exceed that of degussa TiO
2
(P25). In 2007, we [141] prepared Cu
2
O/TiO
2
composites by a simple electrochemical
method, and we coated them on a glass matrix through a spraying method. It was found that the photocatalytic activity of the
Cu
2
O/TiO
2
composite film in the presence of FeSO
4
and edTA was much higher than that for a similar system with only TiO
2
and Cu
2
O film, respectively. Without the presence of FeSO
4
and ethylene diamine tetraacetic acid (edTA), there is no degradation
of methylene blue. As can be observed from Figure 3.11, the Cu
2
O/TiO
2
composite film with the ratio of TiO
2
: Cu
2
O = 1 : 1
(w/w) has much higher activity for photodegradation of methylene blue under VL than that of the TiO
2
, Cu
2
O control film, and
the composite film with only 3 wt.% Cu
2
O, respectively. Furthermore, the photocatalytic activity of the composite film with
only 3 wt.% Cu
2
O is slightly better than that of the TiO
2
and Cu
2
O film. Thus, there should be a synergistic effect in the
composite film. Additionally, the ratio of TiO
2
and Cu
2
O for the composite is critical for the activity of the composite film. The
mechanism for the great improvement of photocatalytic activity of the TiO
2
/Cu
2
O composite film is proposed by the VB theory.
Photodegradation of organic pollutants by a semiconductor is usually completed by photoexcited electrons and holes together.
Thus, it is important to improve quantum efficiency by effective electron and hole separation.
The band edge positions of TiO
2
and Cu
2
O are presented in Figure 3.4. The internal energy scale is shown on the right for
comparison to normal hydrogen electrode (NHe). It is well known that the band gap of TiO
2
is about 3.2 eV and the CB of TiO
2