Environmental Engineering Reference
In-Depth Information
activity of Cu
2
O have been carried out. The reasons Cu
2
O is considered a potential solar-driven photocatalyst are as follows:
(i) it is nontoxic; (ii) it is composed of two very abundant elements; (iii) it has a good absorption coefficient for light with wavelength
above 580 nm [33] and high theoretical solar cell conversion efficiency (up to 18%) [34]; and (iv) it is easy to prepare. However,
it is well known that Cu
2
O can easily be deactivated by photocorrosion [35], although it was reported that photocatalytic
splitting of water on Cu
2
O powder proceeded without any noticeable deactivation for more than 1900 h [30]. Stability of Cu
2
O
has been studied by some researchers [36-41]. It is very clear that Cu
2
O can be oxidized easily if photogenerated holes cannot
be scavenged efficiently because it is thermodynamically favorable for photogenerated holes to oxidize Cu
2
O to CuO. In order
to improve stability and photocatalytic activity of Cu
2
O, numerous efforts have been undertaken on Cu
2
O modification for its
application in the degradation of organic pollution, photocatalytic disinfection, and photosplitting of water. In this chapter,
based on the work done by other groups and in our lab, Cu
2
O-based nanocomposites for environmental protection have been
reviewed for disinfection and degradation of organic pollutants, with a focus on the structural characteristics of Cu
2
O and its
modification, the relationship between structure and photocatalytic activity, and photocatalytic mechanism.
3.2
struCtural feature aNd Cu
2
O mOdifiCatiON
3.2.1 structural feature of Cu
2
O
Cu
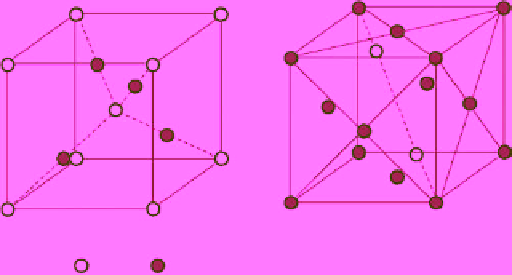
2
O has a very simple structure that crystallizes in a high-symmetry cubic lattice, as shown in Figure 3.1 [42]. The crystal
structure of Cu
2
O is called the cuprite structure [43], which can be viewed as two sublattices, a body-centered cubic (bcc)
sublattice of oxygen anions (Fig. 3.1a), and a face-centered cubic (fcc) sublattice of copper cations (Fig. 3.1b). The oxygen
atoms occupy tetrahedral interstitial positions relative to the copper sublattice, a simple cubic Bravais lattice with the symmetry of
the 224th space group (,
4
3 , so that oxygen is tetrahedrally coordinated by copper. However, copper is linearly coordi-
nated by two neighboring oxygen atoms. These low coordination numbers are very unusual for metal oxides and only two other
substances—Ag
2
O and Pb
2
O—have this crystal structure. Also, a short copper-oxygen bond length in Cu
2
O is unusual, which
is strongly suggestive of metal-metal (Cu-Cu) bonding.
In order to gain a deeper insight into the chemical bonding of Cu
2
O, Gordienko et al. [44] proposed the mechanism
responsible for the formation of Cu-O bonds and possible direct Cu-Cu bonds. The d states of the metal hybridize not
only with the p states of the anion but also with the s and p orbitals of the metal itself. The elucidation of the mechanism
responsible for s-d and p-d hybridizations is of particular importance in explaining the mechanism of chemical bonding
between atoms with filled d shells. As mentioned earlier, Cu
2
O has a cuprite structure, in which oxygen is tetrahedrally
coordinated by copper, whereas copper is linearly coordinated by two neighboring oxygen atoms. The linear coordination
of copper to oxygen and the short interatomic distances in crystals cannot be explained by simple models of interaction
between O
2−
and Cu
+
ions with a completely filled 4d shell but can be interpreted only if it is assumed that copper atoms
form a direct Cu-Cu bond. Several experimental and theoretical works have been dedicated to the study of the electronic
structure of Cu
2
O [45-48]. As shown in Figure 3.2, the valence band (VB) of Cu
2
O is mainly formed by Cu 3d levels,
together with a small contribution from 4s orbitals, and the hybridization of Cu 3d levels and empty Cu 4s levels forms
the conduction band (CB) [49]. The same parity of the bands suggests that Cu
2
O is a semiconductor with a direct gap at
OPnm
h
)
(a)
(b)
O
Cu
figure 3.1
Unit cell of cuprous oxide lattice (cuprite structure): (a) origin on an oxygen site; (b) origin on a copper site. dashed lines
represent the bonds whereas solid lines are only a guide for the eyes. This structure contains two Cu
2
O formula per unit cell [42].