Environmental Engineering Reference
In-Depth Information
As a material for electrocatalytic oxidation of azo dyes, TDNs show null or very low capability to oxidize them [80]
.
However, when electrochemical oxidation is combined with UV irradiation, degradation becomes more effective as in the case
of methyl orange degradation reported by Zhang, who achieved a degradation efficiency of 56.3% for an irradiation time of
90 min with an initial dye concentration of 2 × 10
-4
M [80].
Also, in electrocatalysis, TiO
2
nanotubes have been used as a substrate to support electroactive materials like noble metals
(Ag, Au, Pd, Pt, and Ru) [81-83] or metal oxides. As mentioned earlier, TiO
2
itself is a widely used material in electrochemistry
due to its semiconductive properties, insertion host capacity, and long-term stability [81]
.
These characteristics make TDNs
very suitable to hold other materials providing a large surface area and a chemically stable structure that can be repeatedly used
[83]
.
Gold nanoparticles (3 nm) loaded on TDN layers have proven to be more effective for the reduction of oxygen than gold
nanoparticles dispersed on a compact flat TiO
2
film. Gold as well as other noble metals like silver, palladium, platinum, and
ruthenium have been deposited on TDNs in order to enhance TiO
2
PC properties [81]
.
2.7
Photocatalysis oN tio
2
NaNotubes
In recent years colloidal and particulated TiO
2
have been used to photodegrade pollutants in both liquid and gas phases.
However, these suspended systems must face three inherent technical problems: (i) separation or filtration after the reaction, (ii)
particle aggregation, and (iii) the problems associated with continuous flow systems. Methods to prepare TiO
2
on a solid
support have been reported, but the efficiency of the immobilized system is much lower than that of suspended ones because of
the reduction of surface active area on the immobilized catalyst. Therefore, TiO
2
nanotubes might overcome this drawback due
to its high specific surface area [84]
.
Toward this end, the PC activity of TDNs toward methyl orange discoloration was evalu-
ated and compared with that of a TiO
2
nanoparticle film. In this study, the authors reported a more efficient degradation activity
on the nanotubes than on the nanoparticle film. This higher efficiency is attributed to a more effective separation of the photo-
generated electron-hole pairs and the higher inner surface area of the nanotube structure [84]
.
In general, in order to enhance TiO
2
PC activity, a deposit of noble metal particles increases electron-hole separation and
promotes electron transfer processes. It has been stated that nanotubes loaded with gold and silver nanoparticles form local
schottky junctions that present a higher potential gradient than at the TiO
2
/electrolyte interface [82]
.
Photocatalysis can be
improved if two metals are simultaneously used to modify TiO
2
as it was investigated for a Au-Pd-comodified TiO
2
nanotube
film (Au-Pd-TiO
2
). The activities of naked TiO
2
and Au-Pd-TiO
2
toward malathion degradation were compared. The study
showed that 73.8% of malathion was removed in the presence of naked TiO
2
, but 98.2% malathion removal was achieved when
Au-Pd-TiO
2
was used instead of naked TiO
2
[83]
.
As we can see, one of the major trends in the environmental use of TiO
2
nanotubes is combining them with another material,
metal, or semiconductor in order to enhance their PC properties or to obtain a dual material that has the properties of both parent
materials. In some cases, the TiO
2
nanotube array plays the role of a substrate, which has a large surface area, good mechanical
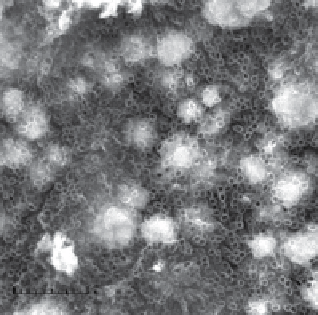
resistance, and greater adsorption capacity to support a highly electroactive metal oxide such as PbO
2
(fig. 2.3a) or snO
2
doped
with sb (fig. 2.3b) [85-87]
.
PbO
2
and snO
2
are known to have a high oxygen evolution potential, which makes them more efficient for electro-oxidation
of organic pollutants. These active oxides loaded on ceramics, Ti, and other support materials are suitable for environmental
(a)
(b)
2 µm
5 µm
figure 2.3
(a) PbO
2
deposited on TDN; (b) sb-snO
2
deposited on TDN.