Environmental Engineering Reference
In-Depth Information
80
HQ-APTS-SiO
2
60
HA-APTS-SiO
2
40
20
SiO
2
0
0
2
4
6
8
10
Exposure time (days)
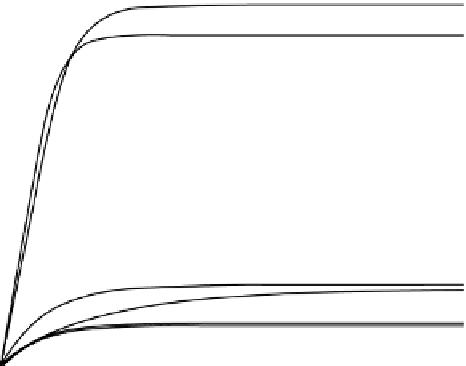
Figure 29.14
Sequestration kinetics of Np(V) in the presence of humic-coated silica gels: leonardite humic acid (HA-APTS-SiO
2
) and
its hydroquinone-enriched derivative (HQ-APTS-SiO
2
); c
0
(Np) = 4.68 × 10
−6
m, pH 4.5, mass/volume ratios for HA-APTS-SiO
2
and HQ-
APTS-SiO
2
are 3.5 and 2.0 g/l, respectively. Reproduced with permission from Ref. [55]. © elsevier B.V.
100
80
pH 3.5
pH 4.6
pH 7.7
60
pH 3.5
pH 4.6
pH 7.7
40
20
0
0
2
4
6
8
Exposure time (days)
Figure 29.15
Sequestration kinetics of Pu(V) by humic-coated silica gel (alkoxysilyl derivative of leonardite HA (HA-APTS-SiO
2
)) at
different pH values; c
0
(Pu) = 4.9 × 10
−9
m, mass/volume ratio for HA-APTS-SiO
2
is 0.345 g/l. Reproduced with permission from Ref. [55].
© elsevier B.V.
Both humic-coated silica gels effectively sorbed Np(V). There was no substantial difference observed in the sequestration
performance of silica gel coated with the parental humic acid versus its hydroquinone-enriched derivative. The amount of
sequestered neptunium was 55% for HQ-APTS-SiO
2
versus 45% for HA-APTS-SiO
2
.
much higher sorption of Pu(V) was observed onto the humic-coated silica gels as compared to Np(V). System equilibrium
was reached after 4 days of exposure. At this time, almost 90% sorption of initial Pu was observed in systems containing humic-
coated silica gels at pH 4.6 and 7.7. Pu sorption onto bare silica gels under the same conditions never exceeded 10%. At the
same time, less Pu sorption was observed at pH 3.5. This result was in line with previous findings of lower Pu sorption under