Environmental Engineering Reference
In-Depth Information
284
286
288
290
292
294
eV
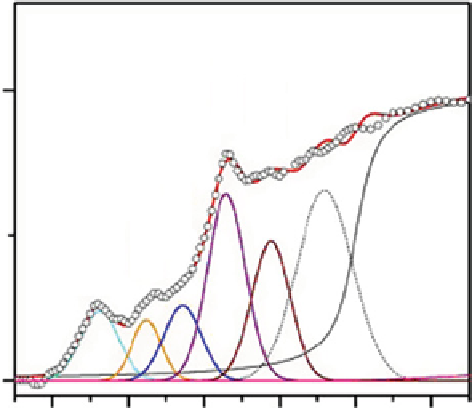
Figure 29.9
Deconvolution fit of cHP-HQ100 with different carbon functionalities as indicated by the labels. The open dots represent
the smoothed measured spectra obtained by cluster analysis and the solid line indicates the fitted spectra.
The “hot spot” produced by the Np-organic matter was observed in the central region of the goethite particle and contained
high amounts of aromatic and aliphatic structures, whereas edge regions of the particle contained lower amounts of these func-
tionalities. The particle's edge structures and the surroundings of the particle itself seemed to be enriched with oxygen-contain-
ing functional groups as deduced from the ratio images of phenol-type and carboxyl-type structures. Similar observations of the
spatial heterogeneity of organic matter functionalities sorbed onto inorganic particles have been reported in the literature for
clay minerals and soil aggregates [40, 41].
Principle component analysis (PcA) and cluster analysis revealed one dominant cluster, whereas the spectral quality of addi-
tional clusters was very poor (not shown here). The cluster spectrum was deconvoluted and quantified, as has been described
elsewhere [42-44]. The spectrum shown in Figure 29.9 is consistent with that reported for fulvic acids from groundwater of the
Gorleben site [45]. At the same time, no organic carbon was detected using carbon K-edge measurements for the sample equil-
ibrated at pH 7.5: a flat line was detected in the energy region 280-310 eV, indicating the optical density (OD) of inorganic
phases (goethite). These findings can be interpreted as representing an absence of adsorption of organic matter.
The conclusion was that the reduction of Np(V) at low pH values in the ternary Np-goethite-HS system was initiated when
hydroquinone-enriched humic derivatives were used. This was not the case with the parent (leonardite) HS. According to
STxm analysis, the modified humics formed surface coatings on goethite colloids at low pH as well as the surrounding cloud
that was rich in organic matter. Such goethite-humic aggregates could serve as effective scavengers for actinides (e.g., Np(V))
from aqueous solutions.
In general, the experimental results discussed here provide positive evidence in support of using quinonoid-enriched humic
materials as agents to facilitate the immobilization of hazardous actinides by inducing their reduction to less mobile forms.
Other potent applications of humic materials could be derived if they could be immobilized as organic coatings onto other solid
supports. These coatings could intercept actinides in their higher-valence state and provide a mechanism for their long-term
stewardship. To solve this problem, another type of modification of humic materials was proposed based on the incorporation
of surface-active silicon-containing groups into the humic backbone.
29.3 Humic nanocoatings anD tHeir use For seQuestration oF mobile
actiniDes in tHeir HigHer-valence state
29.3.1 mineral-adhesive silanized Humic Derivatives and their use for in situ
Preparations of Humic nanocoatings
The original approach to the in situ preparation of humic nanocoatings under aquatic conditions was developed and described
by Perminova et al. [46] and Karpiouk et al. [47]. This approach was based on the use of “mineral-adhesive,” silanized humic
derivatives, which are soluble in water, but undergo a phase switch upon contact with the hydroxyl-carrying surface of the solid