Environmental Engineering Reference
In-Depth Information
reactivities and huge reserves of humic raw materials such as lignite, peat, and lignin, they are not frequently tapped for
remedial needs. This is due to the immense structural heterogeneity that greatly hinders their use in practical applications.
To overcome the issues of structural heterogeneity that define humic materials and also undermine their practical utility
for environmental remediation, an original approach was developed [11] to design humic materials with customized
properties. Based on the previously described roles of HS affecting actinide migration, this new approach must achieve three
goals. First, it must enhance the redox capacity of HS and consequently enable reduction and immobilization of the most
mobile actinide species. The second goal requires that the method enhance the surface activity of HS for creating humic
coatings on solid supports for interception and long-term stewardship of water-borne actinides. This requires a comprehensive
study of interactions between designed humic materials and actinides in the higher-valence states. The third goal assumes
that novel environmental protection technologies will be suggested based on the use of these designed humic materials and
their nanocoatings.
29.2
reDox-enHanceD Humic materials anD tHeir interactions witH actiniDes
Reported values of formal electrode potentials for HS from different sources vary from +0.15 to +0.79V versus standard
hydrogen electrodes [15-20]. This is due in part to their complex makeup. From this range and the reversibility of such redox
transformations, it may be surmised that the redox properties of HS are attributable to the quinonoid moieties present in the
aromatic backbone [21]. moreover, direct electrochemical evidence exists for the quinonoid nature of the redox-active units. It
shows that natural organic matter (NOm) (particularly, the polyphenol fraction) gives an electrode response similar to that of
model quinones such as juglone, lowsone, and anthraquinone disulphonate (AQDS). Hence, similar to quinones, the HS can
participate in both abiotic and biotic redox transformations of contaminants in polluted environments. Several studies can be
cited where HS were shown to participate in abiotic redox transformations of selected actinides. For example, the reduction of
highly oxidized species of Pu and Np (Pu(VI, V) and Np(VI)) has been demonstrated [22-24]. In addition, direct abiotic
reduction of cr(VI) by HS was also reported [25-27]. However, u(VI) and Np(V) reduction was not observed in the presence
of natural HS [7].
Given the discussed importance of quinonoid units for the redox behavior of HS with respect to actinides, we hypothesized
that humic materials with enhanced redox properties can be manufactured by incorporating quinonoid units with known prop-
erties into humic backbones.
29.2.1
Directed synthesis of Quinonoid-enriched Humic materials and assessment of their redox Properties
To realize an approach of enhancing the redox properties of humic materials, the corresponding customized humic materials
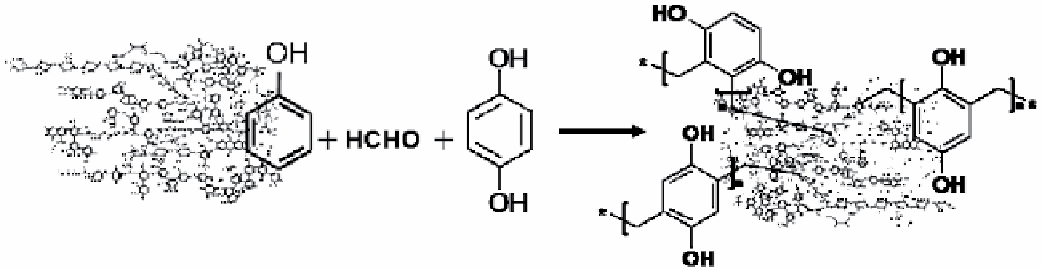
were synthesized by incorporating different quinonoid units as described by Perminova et al. [28] and schematically shown in
Figure 29.3 for the example of hydroquinone.
All modification reactions were run on humic acids isolated from leonardite (oxidized coal). The latter is widely used for
the manufacture of commercial humates. The quinonoid monomers used for the modification of humic materials included
hydroquinone (HQ), catechol (cT), and 1,4-benzoquinone (BQ). The monomers were chosen as ubiquitously being present in
the biopolymers. They were incorporated into the humic backbones at different monomer-to-HS ratios: that is, 100, 250, and
Figure 29.3
Synthetic pathway for manufacturing quinonoid-enriched humic derivatives using phenolformaldehyde polycondensation
(with the example of hydroquinone).