Environmental Engineering Reference
In-Depth Information
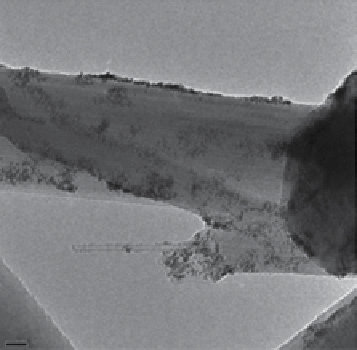
(a)
(b)
20 nm
20 nm
Figure 28.5
TEm images of Pt nanoparticles deposited on (a) carbon nanofibres and (b) carbon nanotubes. reprinted with permission
from ref. [64]. © Elsevier.
oxidation was performed by hu et al. [69]. Uniform and porous graphene nanoflake structures were prepared by Shang et al.
[7] as an excellent support for Pt-based fuel cells. Pt nanoclusters with different thicknesses are deposited on the graphene
surface by the combination of a simple chemical vapor deposition and magnetron sputtering techniques. The hybrid films are
found to exhibit distinctly superior electrocatalytic activities toward methanol oxidation with an ultralow metal loading. Pt nanopar-
ticles supported on nitrogen-doped reduced graphene oxide were investigated by he et al. [70]. As the measurements show, the
catalytic activity of this nanomaterial in the oxygen reduction reaction is practically unchanged, indicating its excellent stability.
Nanostructured thin film (NSTF) catalysts are the leading electrode technology at present for polymer electrolyte fuel cells
because of their significant advantages in durability and specific activity. This type of catalyst consists of a single layer of
crystalline organic whiskers of about 1 µm in length, about 50 nm in diameter, and with a number density of 5 × 10
9
cm
−2
[71].
These whiskers are obtained by temperature annealing of a vacuum-deposited organic red pigment. The deposition of Pt or Pt
alloy thin films on these support structures are performed by the physical vapor deposition or sputter deposition techniques.
These crystalline whiskers have high thermal, chemical, and electrochemical stability under fuel cell operation conditions
[72]. The roughness of the catalyst coating on the whiskers can vary from very smooth to very rough, and it depends on the
deposition conditions and the metal elements.
28.5
NaNostruCtured membraNes
Nanostructured materials are also used in the development of cheaper and more efficient membranes for fuel cells. most
commercial membranes are based on the state-of-the-art hydrated perfluorosulfonic acid material under the commercial name
of Dupont Nafion [73], which combines high ionic conductivity and low gas permeability with good chemical, mechanical, and
thermal strength. however, these fluorine-containing membranes are expensive and don't operate efficiently at temperatures
below 0°C or above 80°C due to the evaporation of water from the membrane structure. For practical applications, the operation
of fuel cells at temperatures above 100°C is desired, both for hydrogen and methanol-fuelled cells, which requires the development
of new classes of membranes. An overview of the research and development of nanostructured membranes for different fuel cell
applications is given by Thiam et al. [74].
The first approach to improve the membrane properties is the use of nanocomposite hybrids. The addition of nanocrystal-
line ceramic oxides to Nafion [75] enhances the membrane characteristics, allowing its operation up to 130°C, when the cell
is fuelled with hydrogen, and up to 145°C in the DmFCs. A new ceramic-based reinforcing porous substrate for fuel cell
applications was developed by Seol et al. [76]. it consists of hygroscopic silica (SiO
2
) nanoparticles interconnected by
3-glycidoxypropyltrimethoxysilane (GPTmS)-based silicate binders and a poly(paraphenylene terephthalamide) (PPTA)
nonwoven support. This unusual ceramic substrate is featured with strong mechanical strength, well-developed nanoporous
structure, high hydrophilicity, and good water retention capability.
Carbon nanostructures are widely studied at present for the purpose of their use in membrane material preparation. The
effect of sulfonic acid-functionalized graphene oxide nanosheets was investigated by Zarrin et al. [77] as inorganic fillers in a