Environmental Engineering Reference
In-Depth Information
28.2
Fuel Cell VehiCles
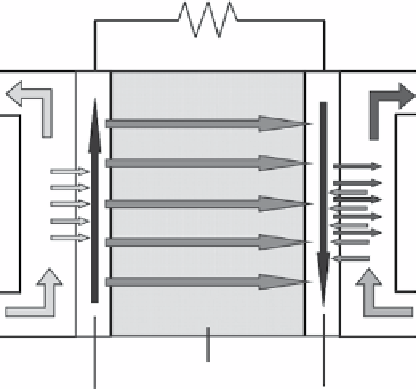
PEFC is the device that generates a current in a set of electrochemical reactions. The working principle of this device is shown
in Figure 28.1. On the anode side of the fuel cell, molecular hydrogen diffuses to the anode catalyst, where it dissociates into
protons and electrons. The protons are conducted through the electrolyte membrane to the cathode whereas the electrons flow
through the external electrical circuit because the membrane is electrically insulated. On the cathode side of the fuel cell,
oxygen molecules react with the electrons and protons on the cathode catalyst, with the formation of water molecules. The
corresponding electrochemical reactions within the fuel cell can be written as follows:
Anode side: h
2
→ 2h
+
+ 2e
−
Cathode side: 2h
+
+ 2e
−
+ 1/2O
2
→ h
2
O
Overall reaction: h
2
+ 1/2O
2
→ h
2
O
Proton exchange membranes are the key components in the fuel cell system. researchers strive to reach the proton exchange
membrane with high proton conductivity, low electronic conductivity and low permeability to fuel, low electroosmotic drag
coefficient, good chemical and thermal stability, good mechanical properties, and low cost [15]. Attempts to understand the
complex and interrelated transport and electrochemical phenomena inside the membrane have activated the appearance of
numerous theoretical and experimental investigations. Numerical simulations have been used to analyze and compare the
effects of fuel cell orientation, operating conditions, and geometry parameters on the cell performance [16-18].
A subcategory of PEFCs is direct-methanol fuel cells (DmFCs), in which methanol is used as the fuel. These devices can be
recognized as an appropriate solution for mobile power sources since they operate at low temperatures with a high-energy
density of methanol that is stable liquid at all environmental conditions [19]. The reactions at the electrodes and overall cell
reaction in this case are as follows:
Anode side: Ch
3
Oh + h
2
O → CO
2
+ 6h
+
+ 6e
-
Cathode side: 3/2O
2
+ 6h
+
+ 6e
-
+ 1/2O
2
→ 3h
2
O
Overall reaction: Ch
3
Oh + 3/2O
2
→ CO
2
+ 2h
2
O
methanol on the anode side of the fuel cell is usually found in a weak solution, because in high concentrations it has the tendency
to diffuse through the polymer membrane and react with molecular oxygen from the cathode side (the so-called crossover effect).
A comprehensive review of the state-of-the-art studies of mass transport phenomena in DmFCs is presented by Zhao et al. [20].
Any hydrogen-fueled PEFC system has four major subsystems, namely, the fuel cell stack, air supply, hydrogen supply, and
thermal and water management system. Figure 28.2 shows the schematic diagram of such a system for vehicle applications [21].
e
-
e
-
e
-
e
-
Excess H
2
H
+
H
2
O+heat
H
2
O
H
2
O
2
H
+
H
2
O
2
Proton
exchange
membrane
Cathode
Anode
Figure 28.1
Working principle of a fuel cell. reprinted with permission from ref. [10]. © Elsevier.