Environmental Engineering Reference
In-Depth Information
(a)
Mn
O
Mn
O
Mn
O
Mn
O
Mn
O
Mn
O
Mn
O
O
Mn
O
Mn
O
Mn
Ca
Mn
O
O
Mn
O
O
Mn
O
Mn
O
O
Mn
O
Mn
Mn
O
O
Mn
Mn
Mn
O
Mn
O
Mn
O
Mn
O
Mn
O
(b)
Mn
Mn
1.9
O
1.8
3.3
2.7
2.7
3.8
O
O
O
2.7
3.0
3.3
Mn
Ca
3.1
3.6
Mn
Ca
3.4
O
3.3
3.6
3.0
O
2.8
3.3
3.4
Mn
Mn
O
O
2.8
2.7
O
O
Mn
Mn
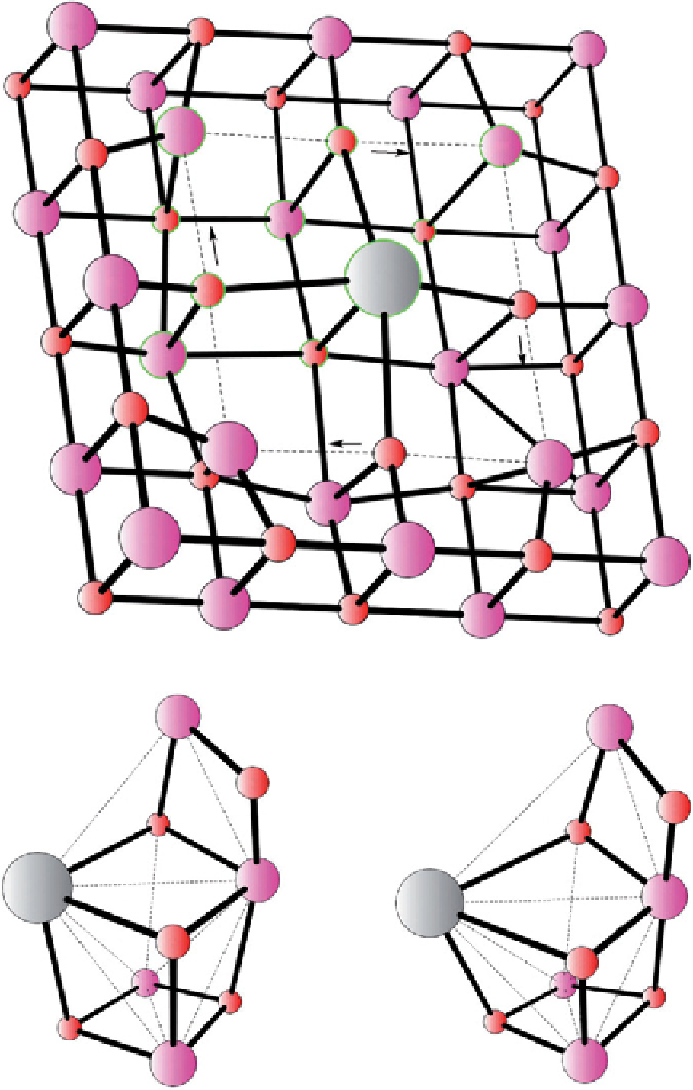
figure 25.4
(a) Siegbahn [57] inserted a calcium ion into the manganese oxide crystal. after optimization, the atoms circled were
selected for the Mn
4
CaO
5
cluster to compare with the WOC in Photosystem II. (b) The cluster to the left is taken out of a mineral after
some relaxation and the right is taken out of the optimized WOC in Photosystem II. reprinted with permission from ref. [57]. © 2011,
John Wiley & Sons.
Multicenters of transition metals such as metal oxides or sulfides can do that, but mono-, and even dinuclear, centers
cannot get involved in such a reaction, because they cannot accommodate and accumulate multielectrons without huge
changes in their structures. Thus, both inorganic cores of enzymes and related inorganic compounds could catalyze
multielectron transfer reactions.