Environmental Engineering Reference
In-Depth Information
and sequester these centers as peptide nests must have an advantage for organisms [21]. another example is that the CaMn
4
O
5
cluster in Photosystem II was derived from a component of a natural early marine manganese precipitate that contained a
CaMn
4
O
5
cluster [21]. It is to be noted that the bond types and lengths of the manganese ions in the tunnel mineral hollandite
(Ba
0.2
Ca
0.15
K
0.3
Mn
6.9
al
0.2
Si
0.3
O
16
) are directly comparable to those in the CaMn
4
O
5
cluster in Photosystem II and can be consid-
ered as both structural [30] and functional [31] models for the CaMn
4
O
5
cluster in Photosystem II; it is also proposed that these
minerals may be assimilated by early cyanobacteria during the archaean period, approximately 3200-2800 million years ago,
to form the cluster in the biological WOC [22, 30].
The third reaction of (nano)minerals with proteins is observed when nanoparticles are formed in the presence of proteins
(Fig. 25.1c) [32-35]. Precipitating agents could be reductants, oxidants, anions, cations, wetting/drying cycles [11], or light that
precipitate metal ions to form particles in the presence of proteins. In this condition, the results show that the protein not only
induces the nucleation, but could also inhibit the further growth of nanominerals, as, without the protein, larger particles are
formed [32-35]. Numerous and very small nanoparticles could form in this condition [32-35].
The dispersivity and stability of nanoparticles in this condition are usually unique. The cofactor formation in particular
enzymes could be considered to be mineralization in the presence of the protein. In other words, one of the numerous and very
small nanoparticles (Fig. 25.1c) produced in the presence of protein could be formed in an appropriate location and be used as
a primitive inorganic core (cofactor). The structure of the primitive cofactor could be changed and modified by amino acid
side groups.
WOC in Photosystem II, nitrogenase, nitrous oxide reductase or oxidase, hydrogenase, and dehydrogenase are enzymes with
an inorganic core, and mineralization could be important in their formation (Scheme 25.1).
It should be considered that there is a tendency to form special structures similar to inorganic cofactors in enzymes
from simple ions (manganese, iron, calcium, and so on) in water even in the absence of any amino acids (Scheme 25.2)
[37-39].
To show the interactions between proteins and minerals, the interactions of birnessite, a common manganese oxide in the
environment, and bovine serum albumin (BSa), a protein that has a strong affinity for a variety of inorganic molecules, are
shown as examples in Figure 25.2.
When birnessite is formed in the presence of BSa, the results show that the BSa not only induces the nucleation, but could
also inhibit the further growth of manganese oxide, as, without BSa, larger particles are formed (Fig. 25.3). BSa also helps to
disperse these nanoparticles in solution and the dispersivity of particles (or minerals) in water can increase the rate of reaction
of particles with other substrates.
The dispersivity of manganese oxide particles in this condition is unique and may be considered a soluble form of nano-sized
colloidal manganese (III, IV) oxide [40].
all these observations show similarities between inorganic cofactors in enzymes and nanoparticles formed in the
presence of proteins. For example, in Photosystem II, Mn
4
CaO
5
is a nano-sized inorganic cofactor [41, 42], and the
authors have proposed that the archaean ocean may have sufficient manganese and calcium ions that, in alkaline condi-
tions, may have enabled protocyanobacteria to assemble oxide mineral as functional compliments of cofactors in
Photosystem II [43].
The hypothesis may be extended to other enzymes with inorganic cofactors (Scheme 25.1) and we may assume the cofactor
formation reaction, in many enzymes, might have been mineralization in the presence of proteins instead of direct adsorption
of minerals onto protein. Many of the inorganic (nano)particles, which could be considered as inorganic cores or cofactors in
enzymes, could have catalyzed related reactions (Table 25.1).
(a)
(b)
(c)
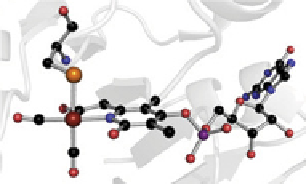
sCheme 25.1
examples of complex inorganic cofactors in biology. Crystal structures of (a) the [FeFe]-hydrogenase H-cluster, (b) the
Mo-nitrogenase FeMo cofactor, and (c) the [Fe]-hydrogenase Fe-guanylyl cofactor (FegP). reprinted with permission from ref. [36].
© 2012, elsevier.