Environmental Engineering Reference
In-Depth Information
prebiotic and simple molecules into biomolecules. For example, Kauffman proposed that the mineral protometabolic surface
could recruit random polypeptides from their environment and improve the efficiency of catalytic activities [19, 20].
russell's group proposed that minerals could attach to proteins to form enzymes [21]. In other words, minerals might be
used initially by polypeptides or carboxylate groups to become active centers of the enzyme precursors. Then, they might
initially catalyze the primary and important reactions of energy conversion and nutrient cycling; for example, pyrophosphate in
successive main chain NH peptide, protoferredoxins as thiolated iron sulfide in peptide, precursors to carbon monoxide
dehydrogenase/acetyl Coa synthetase as nickel sulfide in a peptide, and manganese oxide minerals to the active center of the
water-oxidizing complex (WOC) in Photosystem II [21, 22]. It was observed that there are specific size-dependent interactions
that drive the binding of proteins to nanoparticles [23].
Instead of the “ready-made mineral incorporated into an existing protein theory” proposed by russell's group, the author pro-
poses that the cofactor formation in particular enzymes is biomineralization in the presence of the protein. according to this
hypothesis, mineral-like clusters may form in the presence of a protein and be used as the first inorganic core of enzymes [24].
First, I consider the hypothesis and then I try to explain how the hypothesis could be used to design new environmentally friendly
catalysts.
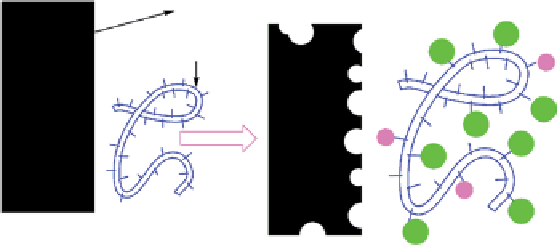
The first and simplest reaction of a protein with nanominerals is the extraction of ions from the minerals by the protein
(Fig. 25.1a). In these reactions, minerals decompose and produce ions that coordinate with amino acid side chains.
The collecting of proteins around (nano)minerals is the second reaction (Fig. 25.1b and c) [26]. an important factor in
these reactions is the surface charge of both protein and particles [26]. For example, Vallee reported that on the surfaces
of titania and silica, carboxylate groups are the preferential peptide-binding groups because of their strong electrostatic
interactions with ions on surfaces [26]. The surface charges of both proteins and oxide particles are largely dependent on
the pH of the solution, and these interactions are governed by physical or chemical forces [27, 28]. It is important to note
that adsorption of a protein on a surface may induce conformation changes in both the biomolecule and on the surface of
the particles [26].
russell and other groups suggested minerals as a “ready-made catalyst” [22, 29]. For example, iron-sulfur centers are poten-
tially important for catalysis via oxidation-reduction reactions since they can exist as Fe
2+
or Fe
3+
, and the ability to stabilize
(b)
(a)
Birnessite
BSA
+
+
(d)
Precipitating agent
(c)
Metal ions
+
Nanoparticle
figure 25.1
(a) an important reaction between mineral and a protein could be the extraction of ions of minerals. (b, c) arrangements of
nanoparticles and proteins depend on their size. (d) When nanoparticles are formed in the presence of a protein, the protein not only induces
nucleation, but could also inhibit the further growth of nanoparticles. reprinted with permission from ref. [25]. © 2013, Springer.