Environmental Engineering Reference
In-Depth Information
before returning it to the ground. The only limitation of this technology is that it takes a long time to achieve cleanup goals [42, 43].
Pump-and-treat systems at some sites with dense nonaqueous phase liquids (DNAPls) may need to operate for considerably
longer periods [43]. Despite the number of limitations exhibited by these systems, pump-and-treat remedies still account for
67% of groundwater remedies proposed or in progress [42, 43].
In the early 1990s, the reducing capabilities of metallic substances, such as zero-valent iron (ZVI), began to be examined for
their ability to treat a wide range of contaminants in wastewater. The most common deployment of ZVI has been in the form of
permeable reactive barriers (PRBs) designed to remediate them [42]. PRBs, first installed at the field-scale in 1994, offer a sub-
stitute for the more established pump-and-treat systems. The first full-scale commercial PRB was approved for use in the state
of California by the San Francisco Regional Water quality Control Board (RWqCB) in 1994 [42]. This passive treatment
system has been used to treat pollutants, including chlorinated hydrocarbons, nitro aromatics, polychlorinated biphenyls
(PCBS), pesticides, and even chromate. The reducing capabilities of ZVI, can dechlorinate chemicals such as TCE and PCBs
[44]. ZVI can reduce hexavalent chromium Cr (VI) to trivalent chromium Cr (III), and precipitate it out of the solution, immo-
bilizing it as Cr (III) hydroxides or chromium-iron hydroxide solid solutions [42, 45].
23.13
nanoscalE zEro-ValEnt iron
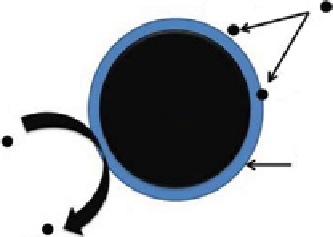
Iron nanoparticles are an attractive component for nanoremediation. Iron at the nanoscale was synthesized from Fe (II) and
Fe (III), using borohydride as the reductant. Nanoscale zero-valent iron (nZVI) particles range from 10 to 100 nm in diameter.
They exhibit a typical core shell structure. The core consists primarily of zero-valent or metallic iron whereas the mixed valent,
that is, Fe (II) and Fe (III) oxide, shell is formed as a result of oxidation of the metallic iron. Iron typically exists in the environ-
ment as iron (II) and iron (III) oxides. nZVI is generally preferred for nanoremediation because of the large surface area of the
nanoparticles and the larger number of reactive sites [46], and also because it possesses dual properties of adsorption and
reduction, as shown in Figure 23.6.
This enables it to be used for the remediation of a wide range of contaminants present
in situ
. Moreover, when ZVI was
allowed greater access to the contamination site, it was found to give out less hazardous waste during the treatment process. ZVI
can also be modified based on the contaminants present. It could be modified to include catalysts like palladium, coatings such
as polyelectrolyte or triblock polymers [47], or be enclosed in emulsified oil micelles [48]. In 2003, nanoscale iron particles
were investigated for their effect on a number of common pollutants in groundwater and contaminated soil. The results showed
that the nanoscale iron particles were highly effective for the transformation and detoxification of a number of pollutants
(Table 23.4). To remove the contaminants, the super paramagnetic property of iron nanoparticles was manipulated and retrieved
using a magnetic field without ignoring its release into the environment. laboratory tests have indicated that more than 99% of
arsenic in water samples can be removed using 12 nm diameter iron oxide nanoparticles [46].
TCE, a hazardous organic contaminant present in water, can be removed by ZVI nanoparticles modified to contain an oil-
liquid membrane. This oil-liquid membrane is generally composed of food-grade surfactants; the biodegradable oil and water
are hydrophobic and form an emulsion with ZVI. This is termed as emulsified zero-valent iron (EZVI) [50]. Since all DNAPls,
such as TCE, are hydrophobic, the emulsion is miscible with the contaminant, allowing an increased contact between TCE
DNAPl and the ZVI present within the oil emulsion droplet [51]. While the ZVI in the emulsion remains reactive, the chlorinated
compounds are continuously dechlorinated within the aqueous emulsion droplet, producing a concentration gradient within the
Me
n
+
Me
n
+
Fe(0)
Me
(
n
-
m
)+
(
n
>
m
)
RCI
FeOOH
RH
figurE 23.6
Schematic diagram of nanoscale zero-valant iron (nZVI).