Environmental Engineering Reference
In-Depth Information
Coupling to individually
synthesized metallic particles
Metal nanoparticle
Initiator on surface
Monomer
Polymer modied w/
Molecule strongly
Interacts w/ metal
Polymer
Coupling by growing metal
nanoparticles in situ
Metallic nanoparticle
Reducing agent
(Electron donor)
Metallic salt
(Ion)
Polymer
Coupling by synthesizing polymer
and metal particle in same reaction
Initiator
(Starts
polymerization and
donates electrons)
Monomer
Metallic salt
(Ion)
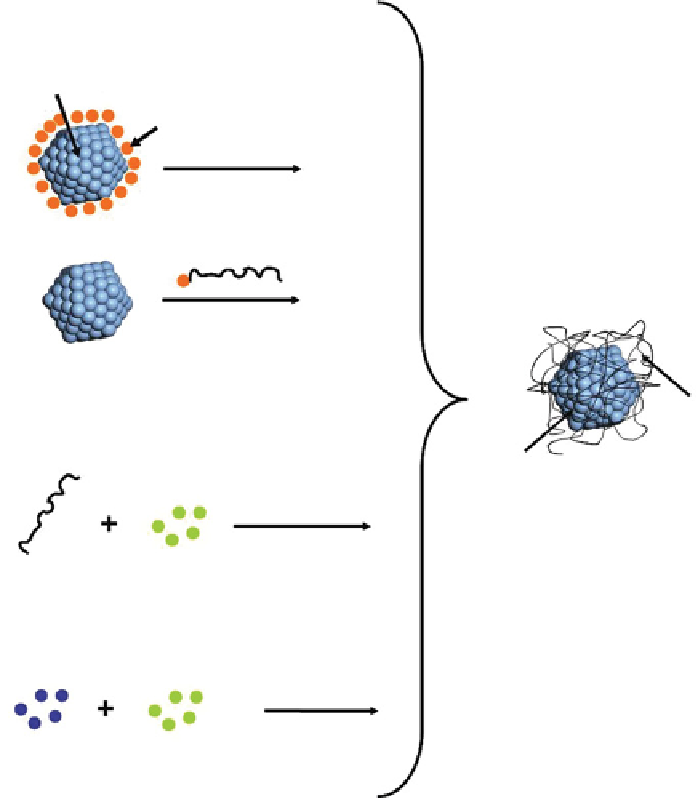
fIgure 22.6
Different mechanisms to couple metallic nanoparticles and polymeric materials are illustrated and labeled respectively. The
first process involves making the metal particles separately and then treating their surface to start polymerization on them or treating a
polymer with functional groups that attach to the metal. The second process involves
in situ
reduction of metal salts in the presence of the
polymer. The last process involves using the initiator as an electron donor and initiator of polymerization; therefore, both polymerization of
the monomer and formation of the particles are performed at the same time. All these lead to the synthesis of coupled polymer-metal nano-
composites with different characteristics.
as well as possible chemical coordination bonds between the polymer and the metal surface. PNIPAM has been previously used
as a capping and stabilizing agent in the synthesis of platinum particles reduced with ethanol [86-88].
Additionally, synthesis of silver [91, 92] and bimetallic platinum-gold [93] colloids has been approached by using simulta-
neous dispersion polymerization of polystyrene and NIPAM in the presence of metallic precursors. The products yield metallic
particles within the PNIPAM grafts on the surface of polystyrene spheres, allowing enhanced catalytic properties and effective
ways of recuperating precious metals. These reports have used X-ray photoelectron spectroscopy (XPs) and Fourier transform
infrared (FTIR) to study the interactions with PNIPAM and silver in these syntheses. They found that the peaks for the carboxyl
and nitrogen hydrogen bonds have shifted, indicating that there is an interaction of silver with these specific groups. This inter-
action provides a potential to develop novel synthesis routes to synthesize colloidal hybrid nanomaterials.
22.7
conclusIons
Current cell culture systems present a challenge of externally controlling the conditions of local microenvironments in order to
emulate
in vivo
-like conditions to study cellular responses to chemical and physical changes in the environment. The current
most successful alternatives to control chemical conditions in microenvironments is the incorporation of responsive mechanical