Environmental Engineering Reference
In-Depth Information
citrate [75, 76], or sodium borohydrate [77, 78]. Previous review papers have discussed several mechanisms in the formation of
metallic particles by wet chemistry methods. The steps described include the creation of metal seeds, followed by the growth
of metallic clusters, and finally agglomeration and further growth of the metallic clusters to form nanoparticles that reach sizes
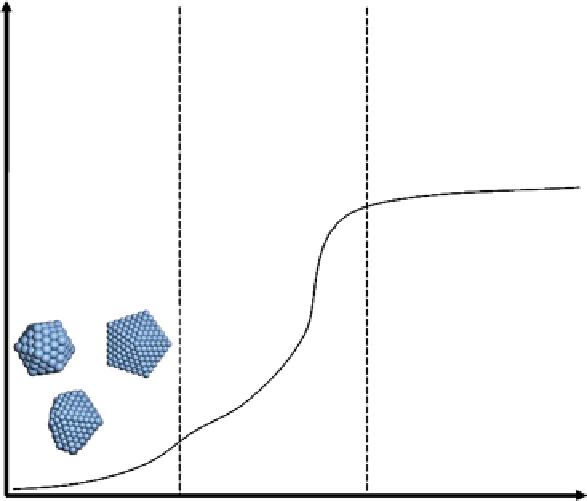
of a few nanometers in diameter (illustrated in Fig. 22.5). The control of the nucleation, aggregation, and growth steps of the
reaction is key in the production of metallic structures with homogeneous sizes and shapes [73, 74, 79]. However, this is not a
thermodynamically stable process since particles in the colloid will tend to minimize their surface energy by agglomerating
with other particles. This leads to the formation of large agglomerates with polydisperse sizes and shapes. size and shape can
be controlled by the addition of a surface stabilizer present in the surrounding solvent during the formation of the metallic struc-
tures. These surface stabilizers are molecules, usually organic polymers that prevent high affinity to the metal surface and the
metallic ions [80, 81]. The final material is then a hybrid polymer-metal nanocomposite where the polymers act as capping
agents; they control the growth of the particles in the synthesis process and prevent surface contact of the particles, which pre-
vents agglomeration during synthesis and storage [63, 81-83].
so far, the most effective and well-studied stabilizing molecules for silver and gold are poly-(vinylpyrrolidone) (PvP) and
sodium citrate [63, 80, 81, 84], respectively. Their interaction with the metals allows fabrication of highly stabilized nanostruc-
tures with controlled shapes and sizes. However, in the production of hybrid materials where coupling of the metallic nanostruc-
ture with a responsive polymer is required, PvP or sodium citrate adhered to the surface must be removed before further surface
conjugation can be achieved. This is done by a ligand exchange process, previously done with thiols [85], using molecules with
stronger bonds to the gold surface than PvP.
various studies have focused on the coupling of polymers and metal nanoparticles to form hybrids. some of the methods
developed (Fig. 22.6) include surface grafting by functionalizing the responsive polymers with thiol groups to promote adsorp-
tion on the surface, and surface-initiated polymerization by prior treatment of the metallic nanoparticle surface with radical
polymerization initiators [75, 76]. Previous reports have observed a strong interaction of molecules that contain chemical
groups with N and o, such as PvP and the amide group in PNIPAM and PAM, with the noble metals, platinum [86-88], gold
[89], and silver [73, 74, 90]. The interaction with the surface of the metallic particles has been attributed to hydrophobic forces
• Reducing agent • Reducing agent
• Prevent seed
formation
Capping agent
• Size seeds
• Stabilize final
nanostructure
• Capping agent
• Stabilize
• Capping agent
high energy
seeds
• Direct growth
of different
shapes
• Stabilize
nanostructure
• Determines
final shape
< 10nm seeds
Seed formation
Growth stage
Stabilizing stage
Time
fIgure 22.5
graphs show the different stages observed for metallic nanoparticle synthesis. The seeding stage consists of the formation
of particles with diameters less than 10 nm. In this stage, the reducing agent plays a major role in the size of the particles depending on how
fast it reduces the metallic salts. The stabilizing agent therefore plays a role in stabilizing different particle shapes of different surface
energies, and the appropriate stabilization leads to the formation of various shapes (spheres, wires, tripods, cubes, stars, etc.). The growth
stage involves agglomeration and growth of small particles. The capping and reducing agent must inhibit seed formation and promote growth,
which leads to homogeneous size and shape distributions. The stabilizing stage only involves the capping agent that is able to stabilize the
resulting metallic nanostructures.