Environmental Engineering Reference
In-Depth Information
(a)
3.0
CNT (
C
i
= 15%)
CNT(APTS) (
C
i
= 15%)
CNT (
C
i
= 50%)
CNT(APTS) (
C
i
= 50%)
2.7
2.4
2.1
1.8
1.5
1.2
0.9
0.6
0.3
0.0
285
300
315
345
Temperature (K)
330
360
375
(b)
1.0
4.5
CNTs
CNTs-TEPA-30
0.9
0.8
0.7
0.6
0.5
0.4
4.0
3.5
3.0
2.5
2.0
1.5
0.3
0.2
0.1
0.0
1.0
0.5
0.0
280
285
290
295 300
Temperature (K)
305
310
315
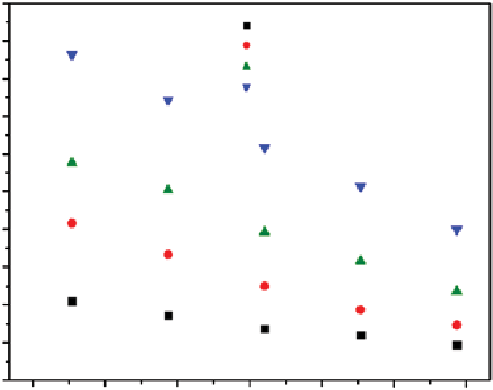
fiGure 21.12
(a) Effect of temperature and CO
2
concentration on adsorption capacity via CNTs and CNT(ApTS). Adapted from Ref.
[38]. (b) Effect of temperature on CO
2
adsorption capacity for CNTs and CT-30 (gas flow rate, 50 ml min
−1
; CO
2
concentration, 2.0 vol%).
Reprinted with permission from Ref. [49]. © 2012, American Chemical Society.
a further increase of temperature to 313°C (physisorption). However, the CNT(TEpA) were capable of both chemical and
physical adsorption [50]. Ye et al. [49] suggested that the increased molecular flexibility of TEpA loaded in the CNT
channels at higher temperatures enables more amino groups to be exposed and be accessible, causing a higher CO
2
adsorption capacity.
Dillon et al. [51] conducted experiments for the adsorption of CO
2
in a covalently attached pEI-functionalized SWCNT
having a different molecular weight than the polymer (600-25,000 Da). The
q
e
increased with the molecular weight of the
polymer (Table 21.1) and decreased with the increase in temperature (Fig. 21.13). Even though the thermal effect in CNT(TEpA)
was not observed by Ye et al. in the presence of the aliphatic polyamine [49], it is suggested that the polymer chain limits the
flexibility and hence the exposure of the amino groups.
The decrease in adsorption with higher temperatures may be attributed to the weakening of the van der Waals force between
CO
2
and the adsorbent surface with a rise in temperature, also suggesting that the adsorption/desorption of CO
2
can be conducted
via a temperature swing operation. In fact, the CNTs and CNT(ApTS) are shown to be thermally stable in air up to 400°C,
which is much higher than the reported desorption temperature of CO
2
from many CNT surfaces (~120°C). For example,
Wilcox et al. conducted the desorption of CNT(ApTS) by heating up to 120°C and passing a flow of pure N
2
.