Environmental Engineering Reference
In-Depth Information
Oxyfuel (O
2
/CO
2
recycle) combustion capture
Steam turbine
CO
2
Electricity
Boiler
Mechanical
energy
CO
2
compressor
Cooling water
Sulfur
removal
Steam
condenser
Cooler and
condenser
Particle
removal
Nitrogen
Water
Gypsum
Fly ash
Fuel
Mechanical
energy
Recycled ue gas
(CO
2
and water vapor)
Oxygen
Air
Air separation
Bottom ash
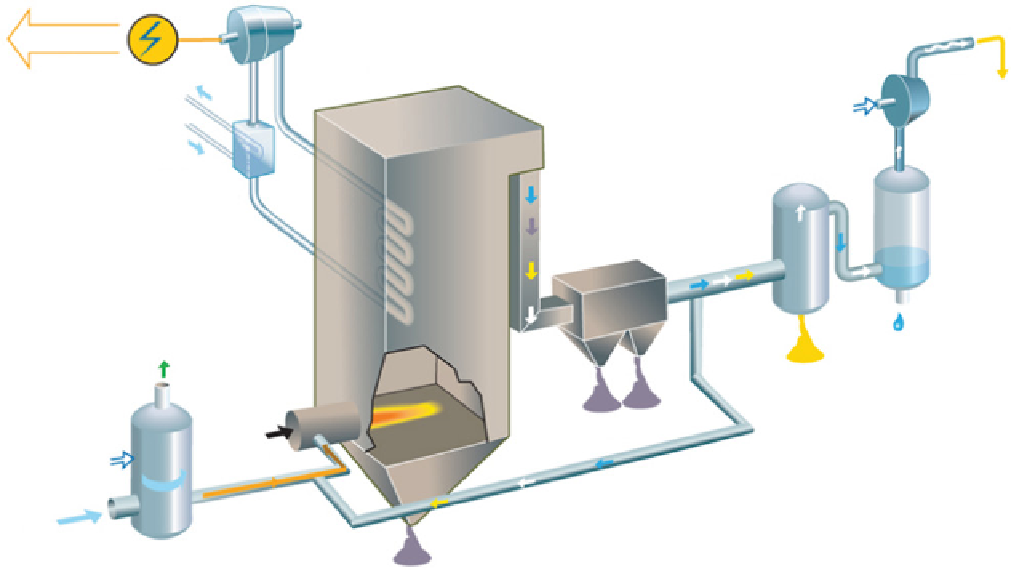
fiGure 21.11
Oxyfuel schematic diagram flow. Courtesy from Ref. [23].
adsorption process might be feasible and the development of a new generation of material that is capable of adsorbing CO
2
efficiently will undoubtedly enhance the competitiveness of adsorptive separation in a flue gas application.
21.3.1.1 Carbon Nanotubes
CNTs are most the most well known among nanohollow structured materials with their dimen-
sions ranging from 1 to 10 nm in diameter and 200 to 500 nm in length. These new materials have unique properties such as
uniform porosity, high pore volume, high specific surface area, and low mass density [37]. CNTs have been proven to possess
good potential for CO
2
capture from flue gas due to their unique physicochemical properties as well as their high thermal and
chemical stability [38]. CNTs are attractive for adsorbing gases because they have high surface area, pore structure, wide
spectrum of surface functional groups, and relatively controllable porosity. The adsorption of CO
2
in carbonaceous materials
such as single-walled carbon nanotubes (SWCNTs) [39] and multiwalled carbon nanotubes (mWCNTs) corresponds to the
amount of CO
2
adsorption which takes place near the carbon surface solid only due to the physical forces (van der Waals
interactions, physisorption) that carbon atoms exert on CO
2
molecules. physisorption occurs due to van der Waals forces
between adsorbate molecules and adsorbents while chemisorption takes place due to chemical interactions between the
adsorbate molecules and the surface functional groups of adsorbents. These nanomaterials have been proven to possess good
potential as superior adsorbents for removing many kinds of organic and inorganic pollutants in air streams [40] or from
aqueous environments [41]. both SWCNTs and mWCNTs have been tested as adsorbents of CO
2
[42]. For example, Lee et al.
[42] compare mWCNTs and granular activated carbon (GAC) for CO
2
adsorption. Under the same conditions, the capacity of
mWCNTs and GAC was 1.57 and 1.65 mmol CO
2
g
-1
sorbent, respectively.
21.3.1.2 Amine Types
At the industrial level, the most common amine compounds used are monoethanolamine (mEA) and
diethanolamine (DEA) [43]. Recently, Chowdhury et al. [44] investigated CO
2
sorption rate, loading capacity, and heat of reac-
tion measurements of 25 amine-based absorbents. Correlating their findings with the differences in chemical structure of the
amines, they suggested that the specific amine moieties and structures used may be selected or modified to tune the CO
2
capture
process. Tertiary amine-CO
2
reaction chemistry suggests that the tertiary amine-CNTs may have high CO
2
capacity with a
relatively low heat of sorption.