Environmental Engineering Reference
In-Depth Information
Furthermore, Bharde et al. identified a group of three proteins that were found to guide the assembly of smaller spherical Au
nanoparticles toward triangular nanoparticles. Such investigations started revealing some of the potential biochemical path-
ways involved in nanoparticle biosynthesis, further leading to the isolation of the metal ion reducing proteins [9]. This
knowledge about the involved biomolecular mechanisms may help promote eco-friendly fabrication strategies for large-scale
synthesis of technologically important nanomaterials.
In addition to employing actinomycete, Sastry's group systematically investigated a range of fungi for the synthesis of metal
nanoparticles including gold, silver, and gold-silver bimetallic nanoparticles. Their initial research demonstrated the ability of
fungus
Verticillium
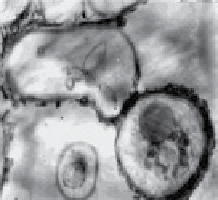
sp. to accumulate Au nanoparticles within the fungal biomass (Fig. 20.2a-d). The accumulation of Au
nanoparticles in the fungal biomass was evident due to the conversion of white fungal biomass to a dark purple color and the
presence of a characteristic Au surface plasmon resonance (SPR) feature at 550 nm in the biomass [56]. The same fungus, on
treatment with Ag (I) ions, also resulted in the intracellular manifestation of Ag nanoparticles (Fig. 20.2e-g) [56, 57]. Although
the mechanism of reduction is not clear, it was postulated that the first step might involve the trapping of metal ions on the sur-
face of cells via electrostatic interaction due to the presence of charged amine and carboxylic groups. These ions are further
reduced to form nuclei that grow to form nanoparticles as more ions are reduced. Further, preliminary experiments showed the
ability of endophytic fungus
Colletotrichum
sp. to successfully yield anisotropic gold nanoparticles of rod-like and prismatic
morphology [58]. Similar to
Verticillium
sp., plant pathogenic fungus
Fusarium oxysporum
was also capable of reducing gold
ions to gold nanoparticles [59] and silver ions to form silver nanoparticles [60]. These biogenic silver and gold nanoparticles
were mostly similar in size typically ranging from 20 to 50 nm in diameter. In addition for its ability to reduce individual metal
ions, when
F. oxysporum
was exposed to equimolar solutions of HAucl
4
and AgNO
3
simultaneously, it resulted in highly stable
Au-Ag alloy nanoparticles of varying mole fractions [61]. The presence of a single SPR feature gradually shifting from nano-
gold to nanosilver plasmon peaks clearly suggested the formation of gold-silver alloy nanoparticles instead of a segregated
metal or core/shell-type structure. Similarly, Nair and Pradeep also showed the ability of different
Lactobacilli
strains to reduce
(a)
(b)
(c)
(d)
(1 1 1)
A
l
(2 0 0)
(2 2 0)
0
400
500
600
λ
(nm)
700
30
35
40
45
50
2
θ
/°
55 60
65 70
1 μm
500 nm
(e)
(f)
(g)
5
4
3
2
1
400
500 600 700 800
Wavelength (nm)
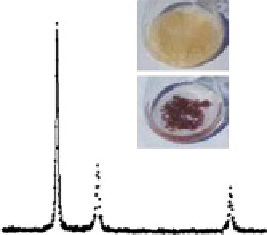
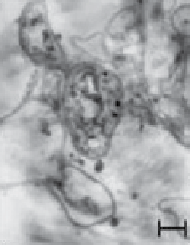
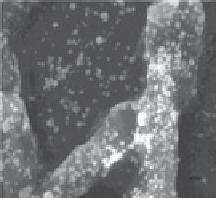
fiGUre 20.2
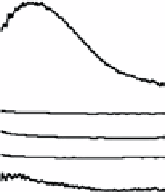
(a) UV-vis spectra obtained from dried biomass of
Verticillium
sp. before (dashed line) and after (solid line) exposure to gold
ions demonstrating the synthesis of Au nanoparticles. (b) XRD pattern of Au nanoparticles from dried biomass of
Verticillium
sp. Inset shows
fungal biomass before (upper panel) and after (lower panel) exposure to gold ions indicating Au nanoparticles within fungal biomass. (c-d)
TeM images of a thin section of fungal biomass showing the presence of intracellular Au nanoparticles. (e) SeM image and (f) TeM images
of
Verticillium
sp. mycelium showing Ag nanoparticles. (g) UV-vis spectrum obtained from
Verticillium
biomass indicating the presence of
Ag nanoparticles. Images reprinted with permission from Refs. [56, 57]. © 2001, Wiley-VcH Verlag GmbH & co. KGaA and © 2001,
American chemical Society.