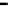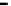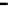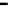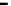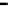Environmental Engineering Reference
In-Depth Information
Charge
Fe
0
(0)
Fe(OH)
2
(2)
Fe(OH)
3
(3)
Discharge
Fe(OH)
x
n
+/-
(-3 to 2)
Layered
Fe(OH)
2
+ Fe(OH)
3
(2-3)
FeO(OH)
n
-
(3 -6)
FeO(OH)
(3)
Overcharge
Fe
n
+
(2, 3)
Fe
2
O
3
(2)
Fe
3
O
4
(3)
FeH
y
n
+
(2, 3)
Fe
x
O
y
n
-
(1 -6)
POM
HPOM
HFeOH
n
+/-
(2, 3)
fiGure 1.2
Fe-Hydrogen Redox Cell: Simplified relationship between n-Fe
0
, Fe
0
products, oxidation number (brackets), and stored
charge in the various ZVM components.
Fe(A
z
)
n
+/-
M(A
z
)
n
+/-
(O
x
H
y
)
-
A
n
-
H
+
M
n
+
H
2
O
Fe
0
Fe
n
+
Fe(O
x
H
y
)
n
+/-
M(O
x
H
y
)
n
+/-
n
e
-
fiGure 1.3
Fe-water Redox cell, simplified sequence of anion and cation exchange.
removal by incorporation into hydroxide/peroxide precipitates of anions and cations, and the reformulation of
organic pollutants into simple alkanes and alkenes [10, 21, 95-98].
4.
Adsorption Model
: ion substitution (Fig. 1.3) of ZVM corrosion products and nano-molecular growth in
self-assembly molecules nucleating around ZVM corrosion products results in the removal (by substitution/adsorp-
tion) of pollutant ions [38-50, 101, 102]. This model is treated in this study as a subset (Figs. 1.2 and 1.3) of the
Galvanic Model.