Environmental Engineering Reference
In-Depth Information
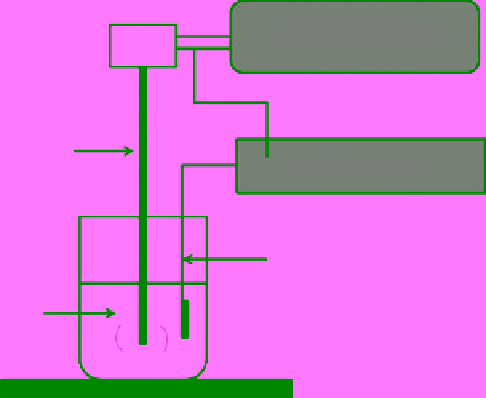
Ultrasound generator
Ultrasound
horn
Electric supply
Counter electrode
Electrolyte
fiGure 18.7
schematic representation of sonoelectrochemical setup used for nanoparticle synthesis.
metals were usually prepared by electrodeposition into the template preformed on the surface of the electrode, but it was diffi-
cult to release the products from the template to obtain nanoporous metal powders. Therefore, those methods could not meet
the requirement for the production of nanoporous metal powders with high yields. Among the electrochemical methods for
nanoporous metal synthesis, sonoelectrochemical reduction is effective since the product can be separated from the cathode and
collected by centrifugation. In this protocol, metal ions were reduced on the cathode surface and dispersed into the solution by
the cavitation effect of the ultrasound. The typical setup for the sonoelectrochemical protocol consists an ultrasonic horn with
electrochemical assembly (Fig. 18.7). In this regard, Reisse et al. first came up with a device for the production of metal pow-
ders by sonoelectrochemical reduction [60, 61]. since then, several kinds of metal and semiconductor nanoparticles have been
prepared using this method [62-64]. Qui et al. [63] discusses PbTe nanoparticles having a rod shape and spherical morphology
(Fig. 18.8). For instance, durant et al. prepared Zn nanoparticles in an aqueous system and studied catalytic activity [65].
In their review article, sáez and Mason reported the nanomaterials that were prepared by pulsed sonoelectrochemistry. The
majority of nanomaterials produced by this technique are pure metals such as silver, palladium, platinum, zinc, nickel, and gold.
The preparation of nanosized metallic alloys and metal oxide semiconductors was also included. A main advantage of this tech-
nique is that the shape and size of the nanoparticles can be adjusted by changing the operating parameters, which include ultra-
sonic power, current density, deposition potential, and the ultrasonic versus electrochemical pulse times. In addition, the effect
of ph, temperature and composition of the electrolyte in the sonoelectrochemistry cell is also possible [66].
18.6.1
Metallic nanopowders
haas et al. prepared copper nanoparticles from an aqueous acidic solution of cuso
4
using PVP as a stabilizer [67]. spherical
copper nanoparticles with a diameter range of 25-60 nm were observed by applying a range of current densities between 55 and
100 mA cm. The first step was the formation of a coordinative bond between PVP and copper ions, forming a cu
2+
-PVP com-
plex. When the current pulse was applied to the formed complex in the solution, the cu was reduced to cu(0) when polyvinyl
alcohol (PVA) was used as a stabilizing agent, and copper with dendritic morphologies was obtained [68].
Zin et al. reported synthesis of platinum nanoparticles from an aqueous chloroplatinic solution [69]. The obtained platinum
nanoparticles were spherical with an average size ranging from 10 to 20 nm. The obtained particles get aggregated into secondary
structures with a mean size ranging between 100 and 200 nm. Tridimensional dendritic Pt nanostructures were prepared when
PVP was used as stabilizer [70].
Aqil et al. prepared gold nanoparticles using pulsed sonoelectrochemistry in an aqueous medium [71]. The aggregation of
nanoparticle was prevented by the addition of electrolyte. The electrodeposition of gold was carried out by applying a potential
in the range of −850 to −1300 mV versus the normal hydrogen electrode (Nhe), and in the presence of α-methoxy-ω-hydroxyl
polyethylene (MPeo) the nanoparticles aggregated and settled down in the electrochemical cell. however, using a MPeo/PVP
polymer mixture, a stable violet suspension was obtained without sedimentation. Most of the nanoparticles are up to the size of
12 nm, together with a few larger particles (30 nm). In the presence of polyethylene oxide (Peo) disulfide polymer, a very stable
suspension of gold nanoparticles with an average diameter of 35 nm was obtained. Jiang et al. synthesized silver nanoparticles