Environmental Engineering Reference
In-Depth Information
OH
-
H
H
O
Fe
2+
Desorption
C
2
H
6
(g)
Cl
-
(aq)
Fe
0
C
2
HCl
3
(aq)
Adsorption
nZVI
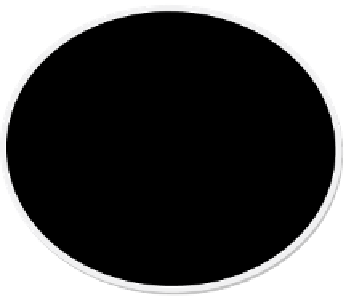
FigUre 17.1
Degradation of Tce by nanoscale iron nanoparticles.
the degradation rate of dichloro-diphenyl-trichloroethane (DDT) was faster with bimetallic ni/Fe nanoparticles than with mono-
metallic nZVI. Their results also revealed that weak acidic or alkaline conditions favor the degradation of DDT.
In real scenarios, however, wastewater contains more than one contaminant. Thus, the effects of competing anions are
significant. Anions, such as bicarbonate (HcO
3
−
), H
4
SiO
4
0
, and H
2
pO
4
2−
, have potential competing agents in As(V) adsorption
[27, 29]. The common divalent metal cations, such as magnesium ions (Mg
2+
) and calcium ions (ca
2+
), present in contaminated
water increase the affinity of nZVI toward As(V) anions. This is attributed to the fact that metal cations cause the nZVI surface
to be more positively charged [26]. Therefore, more experiments are required to investigate the correlation among common
contaminants in wastewater with nZVI adsorption capability.
17.2.1.2 Nanosized Oxides
nanoscale metal oxides rather than microscale metal oxides are preferred as adsorbents due to
the larger specific surface area they cover, which favors adsorption. However, the separation of these nanosized materials from
treated water has also raised some issues. Therefore, nanoadsorbents with good adsorption and magnetic properties have often
been chosen for the said purpose, where separation can be easily achievable using an external magnetic field right after water
remediation. Among nanosized metal oxides, nanosized Fe
2
O
3
, such as hematite (α-Fe
2
O
3
), γ-Fe
2
O
3
, and Fe
3
O
4
nanoparticles,
exhibited magnetic properties.
The ability of α-Fe
2
O
3
, γ-Fe
2
O
3
, and Fe
3
O
4
nanoparticles to remove Orange II, a common azo dye in the textile industry, was
investigated by Zhong et al. [23]. Their results showed that all three adsorbents successfully removed Orange II, with similar
adsorption capabilities. Fe
2
O
3
containing Orange II was also regenerated by catalytic combustion at 300°c in air for 3 h, and the
readsorption performance remained almost unchanged. Furthermore, Hu et al. [21] demonstrated the potential of γ-Fe
2
O
3
nanoparticles as a magnetic nanoadsorbent for the removal of cr(VI) in wastewater. The adsorption of cr(VI) by γ-Fe
2
O
3
nanoparticles proceeded rapidly and reached equilibrium within 15 min, independent of adsorbate concentration. The existence
of competitive ions, such as sodium ions (na
+
), copper ions (cu
2+
), nickel ions (ni
2+
), ca
2+
, Mg
2+
, nO
3
−
, and chloride ions (cl
-
),
in wastewater has no significant effect on the adsorption of cr(VI) by γ-Fe
2
O
3
nanoparticles. Furthermore, cr(VI)-loaded
γ-Fe
2
O
3
nanoparticles were recovered successfully, and were reused in the readsorption of cr(VI) ions.
To increase the adsorption rate of contaminants from water, chang et al. [8, 33] reported the effectiveness of chitosan-bound
magnetic nanoadsorbents using Fe
3
O
4
nanoparticles as cores and chitosan as ionic exchange groups, in the removal of heavy
metal ions, such as cu(II) and cobalt ion (co(II)). chitosan bound covalently on the surface of magnetite was capable of adsorb-
ing metal ions due to the amino groups that serve as chelation sites. The time required to achieve adsorption equilibrium was
1 min for the removal of cu(II) and co(II) ions. The rapid adsorption of heavy metal ions using chitosan-bound magnetite
nanoparticles from aqueous solutions was due to the high specific surface area and the absence of internal diffusion
resistance.