Environmental Engineering Reference
In-Depth Information
N
N
N
N
M
2+
Si
Si
O
O
O
O
O
O
M
O
M
O
N
N
O
M
M
O
Si
N
M
M
O
Si
N
+ M
2+
O
O
O
O
O
Si
Si
OEt
OEt
N
N
N
N
M
2+
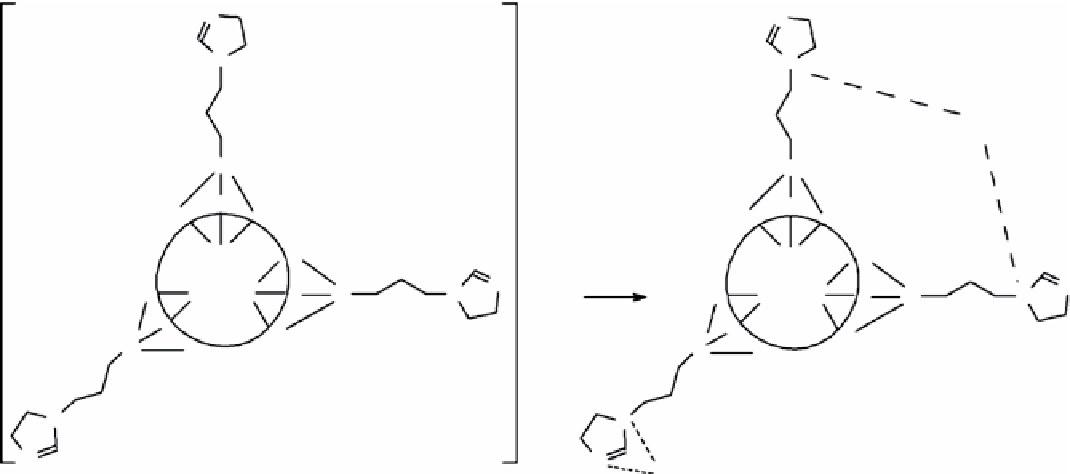
figure 16.5
Reaction between the modified sepiolite and the metal ions.
OH
H
3
CO
O
O
O
+
OH
Si
150 ml toluene
(1)
Si
Si
Si
Cl
Cl
H
3
CO
reflux12 h
OH
OCH
3
BT
CPTS
BT-CPTS
100 ml 95% ethanol
O
O
H
2
N
OH
(1)
+
NH
Si
Si
NH
NH
OH
reflux 24 h
NHED
BT-NHED
figure 16.6
synthesis route of the BT-nHed adsorbent.
A new material has been synthesized using dry process to activate bentonite followed by
N
-(2-hydroxyethyl) ethylenedi-
amine (nHed) connecting chlorosilane coupling agent (fig. 16.6) [77]. The most interesting trait of the new material was its
selective adsorption of rare earth elements. samarium (sm) was quantitatively adsorbed at pH 4 and shaking time of 2 min onto
the new material. under these conditions, the maximum static adsorption capacity of sm(III) was found to be 17.7 mg/g. The
adsorbed sm(III) ions were quantitatively eluted by 2.0 ml, 0.1 mol/l HCl, and 5% Cs (nH
2
)
2
solution.
An efficient adsorbent was synthesized by anchoring n-methylimidazole onto activated palygorskite (fig. 16.7) [78]. The
n-methylimidazole-modified palygorskite was examined as a strongly basic anion exchange adsorbent for the adsorption of
Pb(II) ions from wastewater. The maximum adsorption capacity was found to be 714.29 mg/g at pH 4 and temperature 283 K.
The maximum adsorption capacity of functional palygorskite obtained for Pb(II) ions in this study was found to be comparable
and the highest for all of corresponding clay-related adsorbents reported in the literature.
Hectorite clay has been modified with mBI using homogeneous and heterogeneous routes (fig. 16.8) [79]. due to the
increase in basic centers attached to the pendant chains, the metal adsorption capability of the final chelating materials was
found to be higher than for its precursor. The adsorption of metal ions from aqueous solution follows the order eu
3+
> u
6+
> Th
4+
for all systems. The maximum number of moles adsorbed was determined to be 11.63, 12.85, and 14.01 mmol/g (H
HeT
) for Th
4+
,
u
6+
, and eu
3+
, respectively. The competitive sorption behavior, with variation of pH, was favorable for the separation of
thorium(IV) from binary mixtures with uranium(VI) and europium(III).