Environmental Engineering Reference
In-Depth Information
(a)
Diffusion into
porous
surface
Release of
particle
Analyte particle
Diffusional
surface
Porous sorbent
(b)
Analyte
particle
CNTs
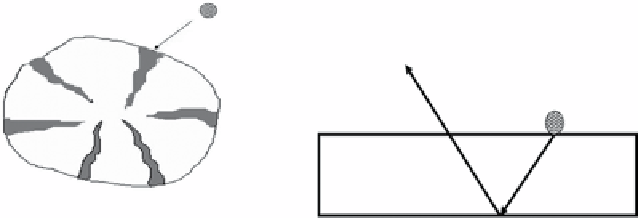
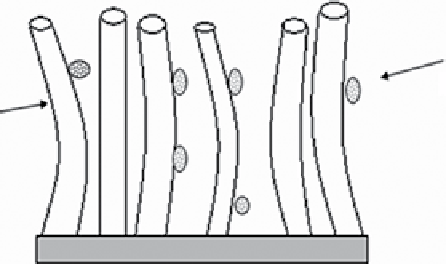
figure 14.5
(a) sketch of a porous carbon sorbent and diffusion into pores, (b) sorption on CNTs for analytes. Reproduced with permission
from Refs. [6, 7]. Ref. [6] © springer and Ref. [7] © science direct Elsevier.
Adsorption on FUls is a subject of increasing experimental and theoretical interest. A few research groups have explored
the adsorption properties of FUls and generally focused on the adsorption of simple gases or vapors of organic compounds on
C60 solid. both types of mechanisms—physisorption and chemisorption—have been realized on FUls both theoretically and
experimentally. some studies have reported interfacial interactions of organic molecules with C60 in aqueous media. FUls
exhibit strong hydrophobicity, and various methods have been used to disperse FUls in aqueous media. These include sonica-
tion, interaction with polymers, redox reactions, and extended mixing [11].
The occurrence of porosity on the FUl surface has been studied via adsorption. some research groups by using Kr, N
2
, o
2
,
and Co
2
adsorption and others by using N
2
and o
2
adsorption proved the existence of micropores in C60. Moreover, research
also pointed out that surface area values normally depend on sample preparation and purification, as well as sample age. More
recent work indicates that FUl powders with higher degrees of purity consist of nonporous particles. overall, the porous texture
of FUls is very sensitive to the synthesis and purification procedures followed in their preparation [7, 12].
The desorption behavior of pyrene, phenanthrene, and naphthalene on FUls and CNTs was examined wherein FUls showed
significant desorption in contrast to CNTs. The reason was the difference in their distinct geometries. spherical FUls resulted
in aggregates providing two space structures, that is, the external surface of aggregates, where adsorption was reversible, and
closed interstitial spaces in small aggregates. Interstitial spaces within small aggregates were unavailable for adsorption, but
these contributed to pore deformation. Rearrangement of small aggregates and penetration of adsorbate molecules into closed
interstitial spaces between small aggregates during adsorption led to molecular entrapment and hysteresis. However, CNTs did
not form closed interstitial spaces in their aggregates due to their length; hence, no adsorption-desorption hysteresis was
observed. Table 14.2 provides applications of FUls as an adsorbent for a variety of analytes [12, 13].
14.4
Cnms for preConCentration of environmental pollutants
14.4.1
solid-phase extraction
solid-phase extraction (sPE) is commonly employed in trace analysis of environmental pollutants because it was demonstrated
to be one of the most suitable methods to preconcentrate the environmental analyte and to eliminate potential interferences. The
advantages of sPE are its high enrichment factor, good recovery, rapidity, the use of small quantities of organic solvents, as well
as the possibility of automatization of the whole process.