Environmental Engineering Reference
In-Depth Information
(a)
(b)
200 nm
200 nm
figure 12.3
Transmission electron microscopy (TeM) micrographs of the nanometric size of (a) La : NaTaO
3
and (b) Sm : NaTaO
3
prepared via the sol-gel method.
table 12.1 Specific surface area and the energy band gap of natao
3
perovskite-type
compounds prepared by the sol-gel method and compared with solid-state compounds
Synthesis method
Material
Surface area (m
2
·g
-1
)
Band gap energy (eV)
Solid state 850°C
NaTaO
3
5
4.0
La : NaTaO
3
5
4.0
Sm : NaTaO
3
5
3.9
Sol-gel 600°C
NaTaO
3
14
4.0
La : NaTaO
3
14
4.0
Sm : NaTaO
3
22
4.0
14,000
H
2
0.6 wt% RuO
2
/La:NaTaO
3
O
2
0.6 wt% RuO
2
/La:NaTaO
3
H
2
1.0 wt% RuO
2
/La:NaTaO
3
O
2
1.0 wt% RuO
2
/La:NaTaO
3
12,000
10,000
8,000
6,000
4,000
2,000
0
0
1
2
3
Time (h)
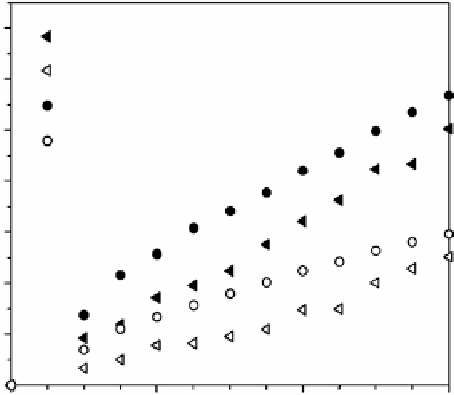
figure 12.4
Hydrogen production using La : NaTaO
3
as a catalyst prepared via the sol-gel method. Reproduced with permission from
Ref. [13]. © 2010, elsevier Limited.
Synthesized samples were tested as a photocatalyst for the water splitting reaction. It was shown that by impregnating
La : NaTaO
3
with Ru, the activity for the water splitting increased; the water splitting activity was almost 25 times greater than
that obtained with the La : NaTaO
3
semiconductor (Fig. 12.4). RuO
2
acts as an electron trap for the excited electrons induced by
UV irradiation, generating very active semiconductors for hydrogen production for water splitting [13].
Results revealed that after 3 h of irradiation, hydrogen production reaches almost 11,500 micromols (µmol). The hydrogen
and oxygen production from the water splitting reaction is shown in Table 12.2. evaluation was carried out using pure water
and a xenon UV lamp of 400 W.