Environmental Engineering Reference
In-Depth Information
1.2
1
0.8
0.6
0.4
0.2
0
0
5
10
15
20
V
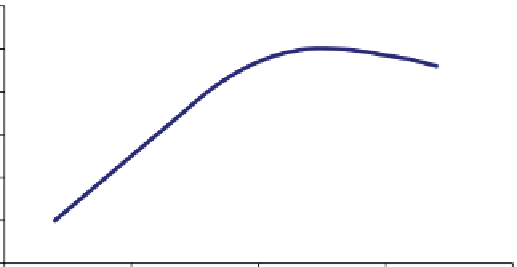
figure 11.1
Dependence of the yield of titanium 6 min after the start of the electrolysis process versus electrolysis voltage.
1
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0
0
1
2
3
4
5
6
pH
figure 11.2
Dependence of the yield of titanium 6 min after the start of the electrolysis process versus ph of the solution.
electrode, which becomes blue. figure 11.2 demonstrates the dependence of
dC
Ti
/
dt
against the concentration of h
2
SO
4
in the
solution 6 min after the start of the electrolysis process. when the ph of the electrolyte comes close to a neutral value, trivalent
titanium is formed on the surface of the Ti electrode.
At the first stage of the electrolysis process when the Ti electrode is the anode, electric current between the electrodes is
about 3-4 mA/cm
2
. In the second stage, after changing the polarity, electric current increases up to 180-200 mA/cm
2
in about
0.1-0.2 s. During the first stage, the oxygen is released on the titanium anode, titanium oxides and sulfates are formed on the
surface, and titanium ions leaving the anode are oxidized by oxygen near the surface of the anode in the solution or react with
NCC that have carboxyl groups as the active groups. The thin semiconductor layer that forms on the surface of the titanium
electrode has high resistance, and the electric current between the electrodes is small, about 3-4 mA/cm
2
. At the same time, the
negatively charged carbon nanoparticles go away from the graphite cathode and functional groups such as carbonyl (>C=O),
hydroxyl (-Oh), and carboxyl (-COOh) groups are formed on the surface of carbon particles.
During the second stage, the oxidation process occurs at the carbon anode. The magnitude of repulsion forces between the
stacked layers of graphite becomes larger than that of van der waals attraction forces between the layers, leading to the formation
of carbon nanoparticles, which changes the polarity of electrodes. The oxides are cleaned from the surface of the titanium
cathode, and the electric current between the electrodes increases up to 180-200 mA/cm
2
. Titanium ions and charged particles
from the titanium oxide interact with carbon nanoparticles and form NCMC (Ti). The oxygen adsorbed on the surface of the
particles forms -Ti(Oh)-O-Ti(Oh)-, which can help the photogenerated holes h
+
to change into a Oh
∙
free radical. Otherwise,
the oxidization activity of Oh
∙
is the strongest in aqueous solution. A typical TEM micrograph of NCMC (Ti) is given in
figure 11.3 and shows that nanoparticles have a spherical morphology. TEM measurements of particles have shown that the
average size of nanoparticles is 6 ± 2 nm.