Environmental Engineering Reference
In-Depth Information
V
-1.5
Mn
2+
/Mn
0
-1
Zn
2+
/Zn
0
-0.5
Cd
2+
/Cd
0
Tl
+
Tl+/Tl0
0
Ni
2+
/Ni
0
Pb
2+
/Pb
0
e
-
B
Conduction band
0
Cu
2+
/Cu
0
0.5
H
3
AsO
4
/H
3
AsO
2
Ag
+
/Ag
0
Hg
2+
/Hg
0
1
Cr
2
O
7
2-
/Cr
3+
PbO
2
/Pb
2+
1.5
Au
3+
/Au
0
2
2.5
h
+
B
Valence band
3
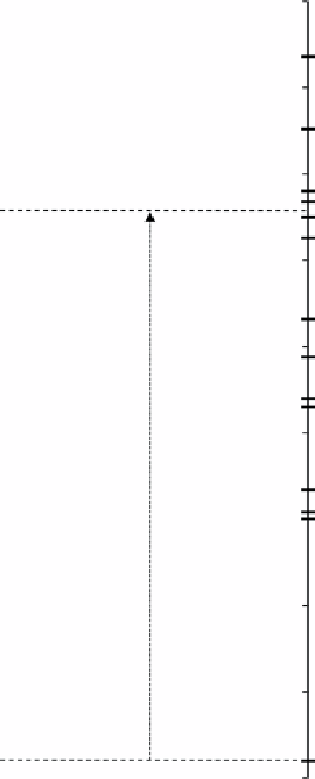
figure 9.1
Position of the reduction potentials of various metallic couples related to the energy levels of the CB and VA of P-25. Adapted
from Ref. [10].
figure 9.1 shows global (multielectronic) reactions, and these can be favorable (exergonic) in principle. However, if one-
electron steps are considered, only thermodynamically allowed reactions would occur. Accordingly, the ion could be reduced
by e
CB
−
−
in a direct reduction step, a reaction that requires the e
CB
reduction potential to be more negative than the one
corresponding to the M
n
+
/M
(
n
−1)+
pair:
n
+
+→
(
−
n
−+
1
MeM
CB
(9.9)
Alternatively, oxidation of the metal ion can occur by reaction with holes or HO
∙
, reaching a higher oxidation state:
n
+
+ ++
+ →
•
(
n
1
)
MhHO M
VB
/
(9.10)
Some metallic species (such as Cr(VI), Hg(II), or U(VI)) cannot be transformed to a higher oxidation state, but they
can be directly reduced by e
C
−
[11]. This process can be accelerated and even produced in thermodynamically not fea-
sible conditions, that is, when the redox potential of the couple to be reduced is more negative than the level of e
C
−
, by
the addition of sacrificial donors to the solution. This donor-mediated reduction of the metal or metalloid constitutes an
indirect reduction process. The donors can be categorized into two different groups: direct h
VB
+
acceptors (as in eq. 9.3),