Environmental Engineering Reference
In-Depth Information
(b)
(a)
Polymer matrix
CNTs
CNT
(c)
CNTs
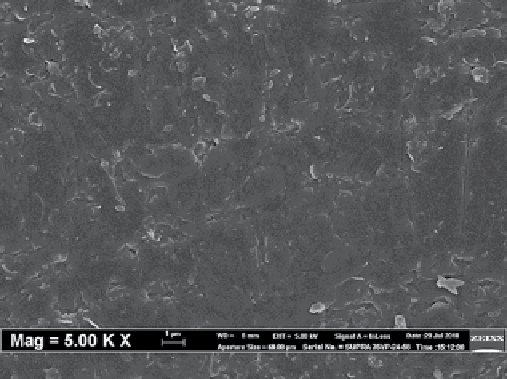
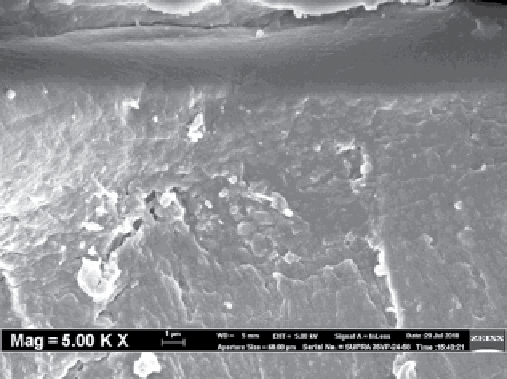
fiGure 8.3
(a) Schematic of CNT-mmms, (b) SEm images of the surface of CNT-mmms, and (c) SEm images of the cross section of
CNT-mmms [103].
Furthermore, considerable attention has been paid to the fabrication of porous-structured CNT-mmms because of their applica-
tion in nanofiltration and ultrafiltration. The results show that CNT-mmms enhance the water permeability and antifouling properties
of the polymer host matrix. Generally, porous-structured CNT-mmms are prepared by the phase-inversion method. The polymer is
dissolved into a suitable solvent, casted, and then immersed in a nonsolvent, which results in precipitation, eventually leading to the
formation of porous-structure membranes. Interestingly, CNTs may alter the membrane structure during membrane formation. The
morphology and porous structure of CNT-mmms change depending on the amount of CNTs added to membranes [87]. The pore
size and surface roughness increase with increased concentration of CNTs and reach a maximum value at a critical concentration.
Previous reports have indicated that the critical concentration ranges from 0.2 to 5 wt.%. Beyond the critical concentration, the high
density of CNTs increases the solution viscosity and delays the exchange between solvent and nonsolvent, thereby leading to reduced
pore sizes and smoother surfaces [87-89, 92, 105]. The pore size of CNT-mmms is important in controlling the water flux and solute
rejection. larger pore sizes increase water flux but compromise solute rejection. Hence, high water flux and solute rejection can be
achieved by adding the appropriate amount of CNTs [92]. CNTs modified with oxygen-containing functional groups reportedly spon-
taneously migrate onto the surface of the membrane during phase inversion because of their hydrophilic effect [88, 93]. This
phenomenon promotes water permeability by attracting water molecules inside the membrane matrix, which facilitates their passage
through the membrane. The improved surface hydrophilicity also enables CNT-mmms to reduce the occurrence of fouling. A major
problem in membrane separation is membrane fouling, wherein solutes are deposited onto the surface or in the pores of the membrane,
thereby decreasing membrane performance. The tendency of the CNT-mmm surface to interact with a water layer in an aqueous
solution reduces possible interactions with the solute. Antifouling properties of CNT-mmms were studied by Celik et al. [87], who
observed a significant decline in the flux of polyethersulfone (PES) membranes with respect to operating time; nevertheless, the dec-
rement is minimal and reaches a plateau with increased operating time. Vatanpour et al. [92] compared the antifouling performances
of pristine and CNT/PES membranes and found that membrane fouling resulting from bovine serum albumin solution can be reduced
in the presence of CNTs. These results also confirm the importance of surface roughness in the antifouling resistance of CNT-based
membranes. Increased surface roughness may enhance membrane fouling because the foulant can easily be entrapped in the valleys
of membranes with a coarser surface, thereby clogging the valleys. However, the charges supplied by the functional moieties on the
surface of CNTs also contribute to the antifouling property by inducing strong electrostatic repulsion forces to repel charged solutes.
The surface of CNT-based membranes is thus shielded, and blockages caused by the solute deposition are prevented.