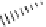Environmental Engineering Reference
In-Depth Information
OH
O
P
OH
OH
HO
O
O
OH
P
P
O
O
O
OH
HO
O
O
O
P
P
O
HO
OH
HO
O
HO
P
O
HO
figure 7.24
Phytic acid.
7.8
NaNoComposites oN the Basis of orgaNometalliC CompouNds
A few organometallics or, much more frequently, products of their decomposition have been used for environmental purposes,
mainly in sensing procedures [62], for instance, [Au
2
Ag
2
(C
6
F
5
)
4
(Nh
3
)
2
]
n
[63] or ferrocene-peptido conjugates [64]. Using
another cene derivative, on titanium basis, hierarchical anatase TiO
2
materials (assembled from very thin TiO
2
nanosheets,
which are composed of numerous highly crystallized anatase nanocrystals) with flower-like morphologies were prepared [65]
via a one-step template-free hydrothermal method starting from titanocene dichloride as precursor and 1,2-ethylenediamine
(eDA) as chelating agent in aqueous solution. These hierarchical TiO
2
materials showed very good photocatalytic performance
when applied to photodegradation of MB, which can be related to the unique features of hierarchical structures, large specific
surface areas, and high crystallization degree of the TiO
2
materials obtained. Organometallic titanium-PANi hybrid materials
(titanium:PANi = from 1 to 5 wt.%) can act as gas sensors (ethanol vapor) at room temperature [25]. To obtain these hybrid
materials, commercially available PANi powder was directly added to organometallic titanium sols, which were synthesized
using the sol-gel method. The sensing mode was based on the variation in the electric conductivity based on the interaction
between the gas molecules and the film. The composite sensors required appropriate ratio to exhibit optimum sensing prop-
erties. Nanocrystalline tin dioxide (SnO
2
) ultrathin films (30-35 nm thick; mean crystallite size ranging from 4 to 7 nm) were
obtained employing a straightforward solution-based route that involved the calcination of bridged polystannoxane films pro-
cessed by the sol-gel process from bis(triprop-1-ynylstannyl)alkylene and -arylene precursors [66]. In the presence of h
2
and
CO gases, these layers led to highly sensitive, reversible, and reproducible responses.
During the past few decades, organometallic methodologies have generated a number of highly effective electrocatalyst
systems based on mono- and bimetallic NPs having controlled size, composition, and structure, used in fuel cells [67]. As an
example, iron, cobalt, and CNF (FeCo-CNF) composite electrocatalysts for the oxygen reduction reaction (Orr) in alkaline
fuel cells (AFCs) were fabricated via electrospinning and the subsequent pyrolysis of a mixture of a nitrogen-containing
polymer and organometallic compounds [68]. The resultant FeCo-CNFs catalysts demonstrated comparable electrocatalytic
activity and stability to commercial carbon-supported platinum (Pt/C) for Orr, a direct four-electron reduction pathway, and
better ethanol tolerance than Pt/C in an alkaline electrolyte.
7.9
CoNClusioNs
A series of nanomaterials on the basis of coordination compounds and few organometallics are currently used for environ-
mental properties. Among others, classic ligands in metal complex chemistry, such as dipyridyl, chitosan, ethylenediamine,
2,6-diaminopyridine, triazene, PANi, phthalocyanines, Schiff bases, salicylic acid, eDTA, amino acids, DTCs, and their