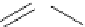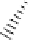Environmental Engineering Reference
In-Depth Information
H
O
H
H
H
N
*
H
H
C
n
O
OH
figure 7.14
Polyglutamic acid.
O
H
2
N
OH
SH
figure 7.15
Cysteine. reproduced with permission from ref. [43]. © 2005, elsevier Science.
Thus, the sorting of Pb
2+
ions in a bloodstream flow was carried out [39] by mixing the ions in a chitosan nanobead aquasolution
and then using a chelating mechanism to selectively sponge them up. To obtain an efficient chelating reaction and chelating
nanobeads that can be attracted and separated from the bloodstream flow, a microfluidic device that had a microchannel with
electrodes was designed and fabricated by a microelectromechanical process. In this device, the microchannel with electrodes
provided a local dielectrophoretic field that was strong enough to manipulate and separate the chelating nanobeads in the con-
tinuous bloodstream flow. Crystalline Cu
2
O NPs were synthesized via the templating method by taking advantage of the chela-
tion of chitosan with copper ions [40]. The resulting Cu
2
O-embedded film exhibited higher photocatalytic activity toward
methyl orange degradation under visible light irradiation. Chitosan NPs were prepared based on ionic gelation between chito-
san and sodium tripolyphosphate and then Ag
+
, Cu
2+
, Zn
2+
, Mn
2+
, or Fe
2+
was individually loaded onto chitosan NPs [41]. Their
antibacterial activities were evaluated by the determination of minimum inhibitory concentration (MIC) and minimum bacteri-
cidal concentration (MBC) against
E. coli
25922,
Salmonella choleraesuis
ATCC 50020, and
Staphylococcus aureus
25923
in
vitro
, showing that antibacterial activity was significantly enhanced by the metal ions loaded, except for Fe
2+
.
7.5.3
amino acids
Amino acids and poly(amino acids) are natural chelating agents for various metal ions. In particular, zinc ions were encapsulated
[42] in situ in a conductive polypyrrole film using polyglutamic acid (Fig. 7.14) as a localized complexing agent within the film.
The subsequent electrochemical reduction of the metal ions to zero-valent metal led to the formation of NPs. An important
advantage of this electrochemical approach is facile regeneration of the particles, as well as prevention of the aggregation of NPs
in the conductive polymeric film. The formed NPs were composed of zinc and were 18 ± 7 nm in diameter. In addition, the NP/
polymer composite was used to reduce halogenated organics, indicating its potential usefulness in remediation applications.
A series of reports are dedicated to cysteine-containing chelating nanomaterials. Thus, an adsorbent for the capture of
mercuric chloride vapor from flue gases on the basis of a chelating ligand on cysteine basis (Figs. 7.15, 7.16, and 7.17) with an
ionizing surface nano-layer on a mesoporous substrate was reported [43]. The maximum theoretical (equilibrium) capacity for
mercury removal was estimated to be 33 mg hg/g, and thermal stability tests indicated stable operation up to 135°C. In addition,
poly-α,β-dl-aspartic acid is well known as the green chelant of various metal ions. To provide a nanochelant for treating Pb(II)
poisoning, poly-α,β-dl-aspartic acid was modified [44] with l-Cys to form poly-α,β-dl-aspartyl-l-cysteine (PDC). dl-Asp
was converted into polysuccinimide through thermal polycondensation, and the amidation of polysuccinimide with l-Cys
provided PDC. In water, PDC formed various porous nanospecies, which benefited the removal of Pb(II). PDC did not remove
the essential metals, including Cu
2+
, Fe
2+
, Mn
2+
, Zn
2+
, and Ca
2+
, in treated mice.
Nanomaterials on the basis of carbon allotropes can also be functionalized with amino acids and used for absorption of
pollutants such as cadmium. Thus, graphene oxide (gO) nanosheets were decorated with a cysteine-rich metal-binding