Environmental Engineering Reference
In-Depth Information
N
N
N
N
N
N
(ClO
4
)
2
Cu
N
N
N
N
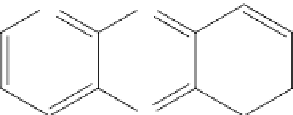
figure 7.7
[Cu(L)
2
](ClO
4
)
2
of Schiff base ligand (L = 4,5,9,13,14-pentaaza-benzo[b]triphenylene).
electrocatalytic properties for the detection of thiocyanate and nitrite in aqueous solutions compared to the bulk FePc counterpart.
Polycopper tetraaminophthalocyanine (CuTAPc) nanowires and nanotubes, fabricated [27] on porous alumina templates by
electropolymerization, showed that their lengths could be controlled by the number of cycles applied and the monomer
concentrations, while their diameters are confined by the pore size of the template. The product of electropolymerization
(whether nanowires or nanotubes) was revealed to be a function of the monomer concentrations. These nanostructures may have
applications in microelectronics, chemical sensing, and catalysis. Copper-tetrasulfonated phthalocyanine (CuTSPc)-sensitized
mesoporous SnO
2
, namely, CuTSPc/SnO
2
(with specific surface area and pore size of 0.1 mol.% CuTSPc/SnO
2
236 m
2
/g and
2.6 nm, respectively), prepared by a template-free hydrothermal method, contained the sulfonated groups of CuTSPc bonded to
the tin ion in a chelating bidentate mode. Its photocatalytic activity and circulating degradation rate (with 0.1 mol.% CuTSPc/
SnO
2
) under low-power visible light (15 W), detected using rhodamine B (rhB) as the objective decomposition substance, were
revealed up to 87% during 180 min of reaction [28].
Among other N-containing ligands that are nanomaterials, we note a solid nanocomposite material on the basis of NPs of a
metal coordination polymer with CN ligands including M
n
+
cations (Ni
2+
, Co
2+
, Mo
5+
, Fe
2+
, or Fe
3+
) {M is a transition metal
and
n
is 2 or 3} and [M¢(CN)
m
]
x
−
anions ([Fe(CN)
6
]
3−
or [Fe(CN)
6
]
4−
, [Mo(CN)
8
]
3−
, [Co(CN)
6
]
3−
) {where M¢ is a transition
metal,
x
is 3 or 4, and
m
is 6 or 8} [29]. These compounds were patented as a method for fixing an inorganic pollutant, such as
radioactive cesium. Schiff bases, classic objects in coordination chemistry, also revealed activities in nanostructured form.
Thus, the Cu(II) Schiff base complex (Fig. 7.7), [Cu(L)
2
](ClO
4
)
2
of Schiff base ligand (L = 4,5,9,13,14-pentaaza-benzo[
b
]tri-
phenylene) was synthesized, and its nanostructured compound was prepared [30] by sonochemical and solvothermal methods.
The free Schiff base and its metal complex were screened for antibacterial activities; the metal complex was observed to be
more active than its free Schiff base ligand. Very high adsorption capacity of trace amounts of Pb(II), Cd(II), and Cu(II) in envi-
ronmental and biological samples was revealed for iron oxide-silica magnetic particles with a Schiff base (Fe
3
O
4
/SiO
2
/L) [31].
According to the authors, this magnetic solid phase is an ideal support because it has a large surface area and good selectivity
and can be easily retrieved from large volumes of aqueous solutions. The detection limits were 0.14, 0.19, and 0.12 µg/l for
Pb(II), Cd(II), and Cu(II) ions, respectively.
7.4
o-CoNtaiNiNg ligaNds
Pure O-containing ligands as part of nanomaterials are represented with fewer examples. Thus, the preconcentration of trace
heavy metal ions in environmental samples, based on the sorption of Cu(II), Cd(II), Ni(II), and Cr(III) ions with salicylic acid
(Fig. 7.8) as the chelate on silica-coated magnetite NPs (Fig. 7.9), was used for evaluating these trace and toxic metals in var-
ious waters, foods, and other samples [32]. These magnetic NPs carrying the target metals could be easily separated from the
aqueous solution simply by applying an external magnetic field; no filtration or centrifugation was necessary. An efficient
method was undertaken [33] for synthesizing highly electrocatalytic Pt NPs on a carbon nanofiber (CNF) material, based on
absorption of Pt(acac)
2
molecules on the functionalized CNFs and their further reduction to Pt NPs (2.9 ± 0.4 nm and 100%
loading yield) by diffusion-limited sublimation in a confined space. The MeOh oxidation current density per mg Pt of Pt-loaded
CNF sample was found to be approximately 60 times as high as in the commercially available sample and superior to other
existing samples. In addition, 3,4-dihydroxy-9,10-dioxo-2-anthracenesulfonate (Alizarin red, Ar, Fig. 7.10) chelates TiO
2
NPs
through the catechol moiety [34]. reduction of Cr(VI) to Cr(V) in the coupled Ar@TiO
2
system utilizes a high fraction of
the photogenerated electrons and induces degradation of the complex. Addition of the chelating polymer polyacrylic acid (PAA,