Environmental Engineering Reference
In-Depth Information
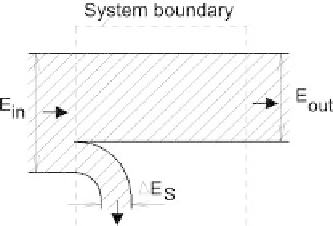
Figure 2.3.1
Bands diagram of energy balance.
Figure 2.3.2
Bands diagram of the traditional exergy balance.
where
B
in
and
B
out
are the respective sum of exergy delivered and released from the
system,
B
S
is the change in the exergy of the system and
δB
is the exergy loss due to
the process irreversibility, calculated from the Guoy-Stodola law, equation (2.2.10).
The bands diagram for exergy balance is shown in Figure 2.3.2. In comparison
with the respective diagram for energy balance (Fig. 2.3.1), the exergy diagram shows
the exergy
δB
disappearing within the system.
Like the energy balance, the exergy balance can be differently tailored depending
on the considered problem and actual conditions. For example, some components of
exergy can be neglected either due to relatively small changes, or because they are
unchanged at all. The balance equation can be written for steady or transient systems,
for system considered on the macro scale or micro scale using differential equations,
etc. Obviously, for calculation of exergies, there is no freedom in defining the reference
state, which is only the environment, as determined by exergy definition.
For any elemental process lasting an infinitely short time, the exergy balance
equation can take the form:
B
in
dt
+
B
out
dt
=
dB
S
+
δB
(2.3.10)
where
B
in
and
B
out
are the respective fluxes of exergy delivered and extracted from the
system and
dB
S
is the total differential exergy growth of the system.

Search WWH ::

Custom Search