Environmental Engineering Reference
In-Depth Information
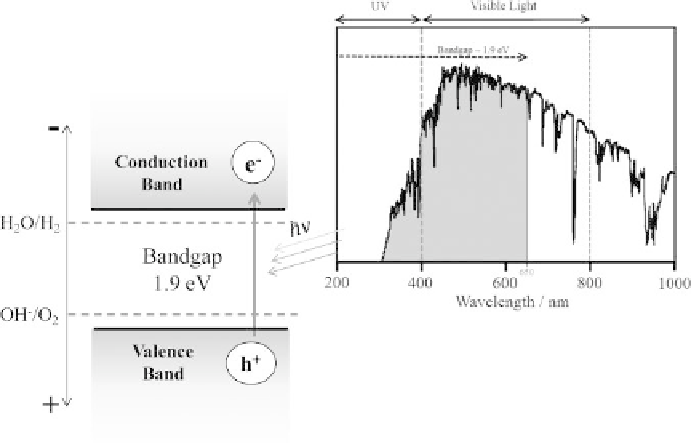
Figure 10.2.5
Schematic illustration of a semiconductor with a hypothetically ideal bandgap of 1.9 eV.
Right: Intensity of sunlight vs.wavelength for AM1.5 conditions. The grey area represents
the part of the spectrum that can be absorbed by a semiconductor with a bandgap of
1.9 eV.
vi
)
The charge transfer from the surface of the semiconductor to the solution must
be selective for water-splitting and exhibit low kinetic overpotentials (Chen et al.,
2010);
vii
)
The material must be sufficiently abundant, harmless and cost-effective.
Since overpotentials are required at various points in the system to ensure suf-
ficiently fast reaction kinetics, i.e. related to the electrochemical reaction kinetics at
anode and cathode and charge transfer (inside the electrodes and in the electrolyte),
the minimum bandgap required to split water is at least 1.9 eV. This value also imposes
that the semiconductor is able to absorb light for wavelengths lower than 650 nm, as
show in Figure 10.2.5 (Krol et al., 2008).
Despite the research efforts to date no single semiconducting material has been
found that will fulfill all the requirements needed to generate standalone devices for
solar hydrogen production from water-splitting (Krol et al., 2008; Sivula et al., 2011).
10.2.3 Materials overview
The keystone in water photoelectrolysis is the development of an efficient, robust, reli-
able, cost-effective, and stable photoelectrode system (Grimes et al., 2008). The first
material recognized to split water under UV light was TiO
2
, reported by Fujishima
and Honda in 1971 (Fujishima and Honda, 1972). Thenceforward, extensive efforts
have been made to find a suitable material for efficient photoelectrodes. Thus, during
the last three decades, different types of semiconductors were studied such as metal
Search WWH ::

Custom Search