Environmental Engineering Reference
In-Depth Information
Table 9.3.3
Distribution of energy requirements of methanol production.
H
2
production: equivalent thermal
CCS: equivalent thermal energy requirement
energy requirement for 3 moles of H
2
for 1 mole of CO
2
Method
kJ
method
kJ
Cu-Cl cycle
1759
Na
2
CO
3
-based
353.0
1759
K
2
CO
3
-based
363.8
1759
CaO-based
440.4
1759
MEA-based
412.0
Water electrolysis
2147
Na
2
CO
3
-based
353.0
2147
K
2
CO
3
-based
363.8
2147
CaO-based
440.4
2147
MEA-based
412.0
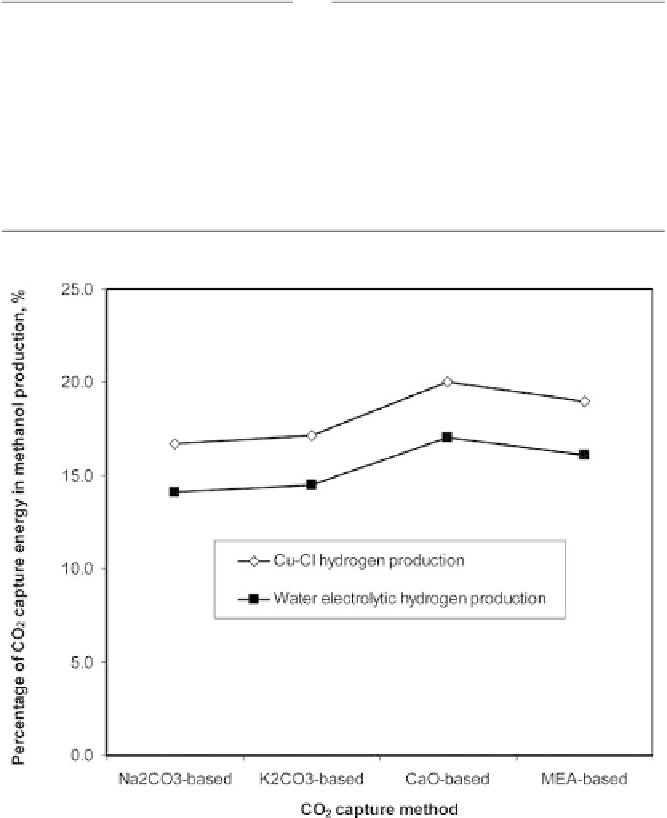
Figure 9.3.1
Percentage of CO
2
capture energy in the synthesis of methanol production.
by hydrogen preparation accounts for the majority of energy cost in the methanol
production process.
As Reaction 9.3.4 can be self-sustained and the solar reactors for hydrogen pro-
duction have been presented in the hydrogen production section of the chapter, this
section will focus on the reactors for CO
2
capture, particularly the extraction of solid
carbonates from the aqueous solution and the release of CO
2
from the carbonates or
sorbents. As the carbonation and calcination operations are very mature in industry,


Search WWH ::

Custom Search