Environmental Engineering Reference
In-Depth Information
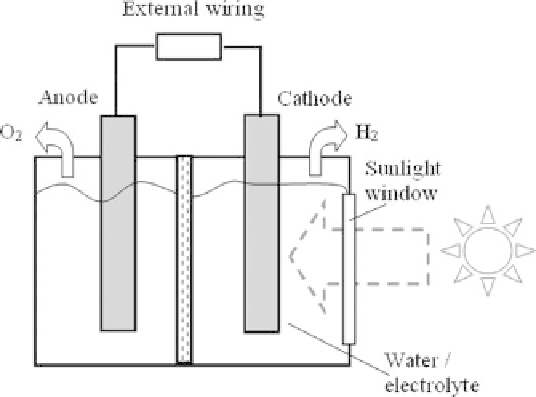
Figure 9.2.5
Components of a photoelectrolysis or photoelectrochemical unit.
Figure 9.2.5 shows the basic components of a photoelectrolysis or photoelectro-
chemical (PEC) hydrogen production unit, including a sunlight absorbing electrode
(typically made or coated with semiconductor) and a counter electrode (typically metal)
immersed in an electrolyte. The sunlight absorbing electrode must be arranged to face
the sunlight window to capture sufficient solar radiation to generate electric poten-
tial. The sunlight absorbing electrode should be wired either externally or internally
with the other counter electrode so as to form a closed circuit. The reaction mech-
anism is described in Equations 9.2.11 and 9.2.12. It can be found that the sunlight
absorbing material has a dominating role in determining the hydrogen production effi-
ciency. The band gap, i.e., the potential, created by the electrode material must exceed
the bottom theoretical limit of 1.23 eV to split water molecules, plus overcoming the
electric resistance of the closed circuit. It was also reported that the materials for
the sunlight absorbing electrode are very likely subject to electrochemical corrosion.
Therefore, many studies are conducted to the development of new anti-corrosion and
high efficiency materials (Grätzel, 2003). Another option to improve the performance
of photoelectrolysis is to utilize external power to boost the electrode potential. This
is a type of hybrid system of water electrolysis and photoelectrolysis. In the hybrid
system, the electrode must be a sunlight absorbing material, so this section suggests
that the system is still viewed as photoelectrolysis rather than water electrolysis due to
the distinct sunlight capturing and electricity generation patterns.
Recently, there is an alternative design of a PEC that makes the entire device a
microparticle, nanoparticle, or nanofibre (Vayssieres, 2009; Solarska et al., 2012;
Yang, 2011; Grimes et al., 2007). The device includes the mini-cathode, mini-anode
and photovoltaic components sandwiched together in single particles that are sus-
pended in an aqueous solution. Hydrogen is evolved at the cathode and oxygen at the
anode. The reaction byproducts, OH
−
and H
+
recombine in the solution completing
Search WWH ::

Custom Search