Environmental Engineering Reference
In-Depth Information
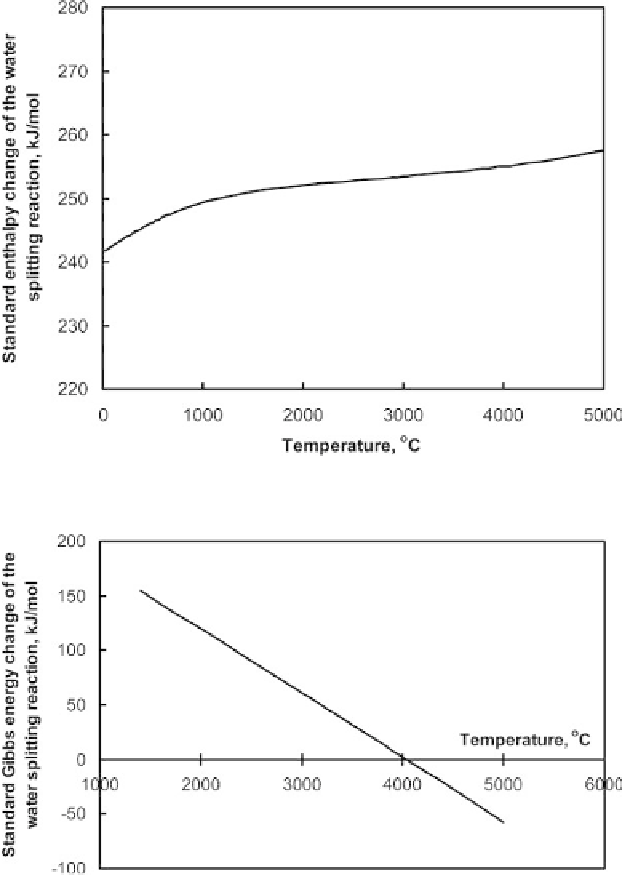
Figure 9.2.1
Standard enthalpy change of water splitting reaction vs. temperature.
Figure 9.2.2
Standard Gibbs energy change of water splitting reaction vs. temperature.
4,000
◦
C, at which the Gibbs energy change becomes negative and the water splitting
becomes spontaneous.
From the Gibbs energy change in Figure 9.2.2, the water decomposition equi-
librium constant can be calculated. Then according to the equilibrium constant, the
water decomposition percentage can be estimated. Figure 9.2.3 shows the direct water
decomposition percentage at different temperatures. It can be found that the below 2%
of water directly split at 2,000
◦
C, and if 40% of water is split, the temperature must
be higher than 4,000
◦
C. This is a very high temperature that would forego engineering
Search WWH ::

Custom Search